Acid Base Balance
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
H+
Hydrogen ion (lost an electron)
Highly reactive
Goal of cell/body: limit the amount of H+
Acid
Any solute that dissociates in solution that releases H+ ions (proton donors)
Strong acids - completely dissociate
Ex: HCl + H2O → H+ + Cl-
Weak acids - do not dissociate completely; release fewer H+
Ex: CH3COOH (acetic acid) < - > CH3COO- + H+
Base
Any solute that removes H+ (proton acceptors)
Strong base
Ex: NaOH + H2O → Na+ + OH
Weak base
Ex: NH3 (ammonia) < - > NH4+ + OH-
pH
Power of hydrogen
Measure the acidity of alkalinity of a solution; presented in a log scale
7 = neutral
Less than 7 = acidic
Greater than 7 = basic
pH scale
pH = -log[H+]
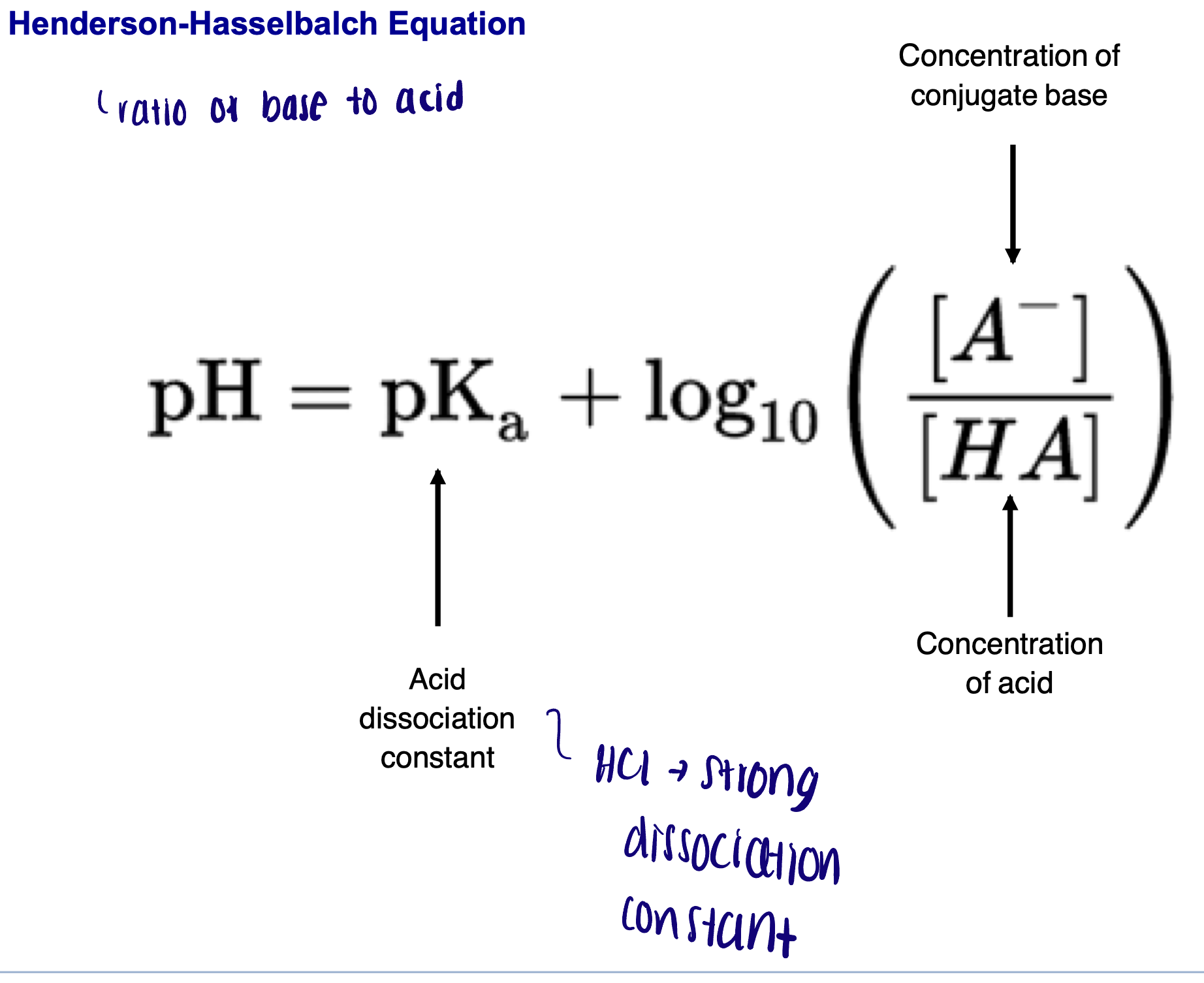
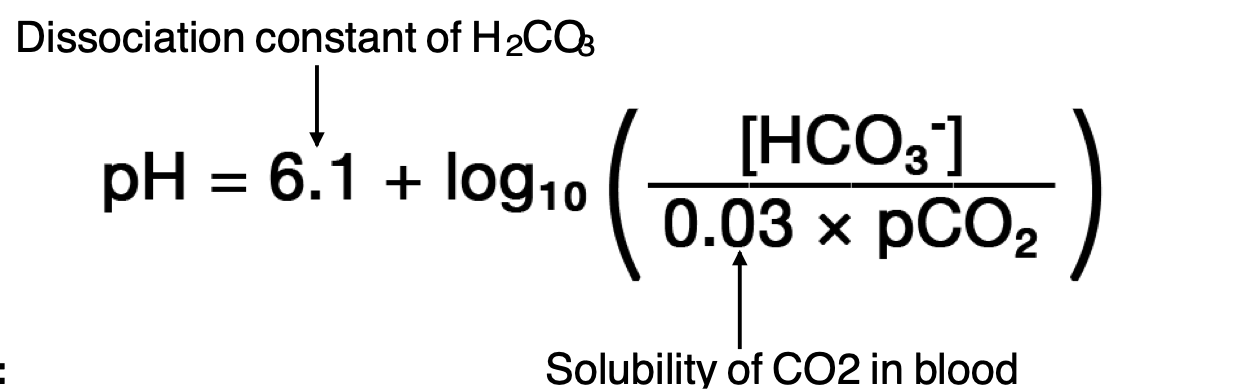
Henderson -Hasselbalch equation
Importance of pH inside the cell
pH of endosome → lysosome
Low pH able to…
Endosome → lysosome (pH = 4.7)
Low pH → able to degrade things
Importance of pH inside the digestive system
Stomach has a low pH (1.5) to help breakdown food
Blood pH
7.35-7.45
Acidosis: Less than 7.35
Alkalosis: More than 7.45
Types of acids
Fixed
Organic
Volatile
Fixed acids
Do not leave solution, excreted via urination
Cannot be excreted via the lungs
Have to rely on kidney
Ex: sulfuric acid (H2SO4) and phosphoric acid (H3PO4)
Organic acids
Metabolic acids (produced via cellular metabolism)
Ex: Lactic acid
Cannot be excreted via the lungs
Voltailte acids
Leaves the body via respiration
Contributes the most to changes in pH
Is excreted via the lungs
Ex: carbonic acid (H2CO3)
How does your body maintain pH
Buffer systems
Breathing (respiratory compensation)
Renal system
What are buffers
Substances that help prevent a drastic shift in pH (decrease the amount of free H+ or OH- that is in the solution
Blood is a natural buffer
Buffer systems
What do they do
Generally consists of
3 major buffer systems and where
Buffers form chemical systems that absorb excess acids or bases and thus maintain a relatively stable pH
Generally consists of a weak acid and weak base
H < — > H+ + Y- (Y= anion that functions as a weak base)
3 major buffer systems:
Phosphate buffer system (ICF)
Protein buffer systems (ICF and ECF)
Carbonic acid - bicarbonate buffer system (ECF)
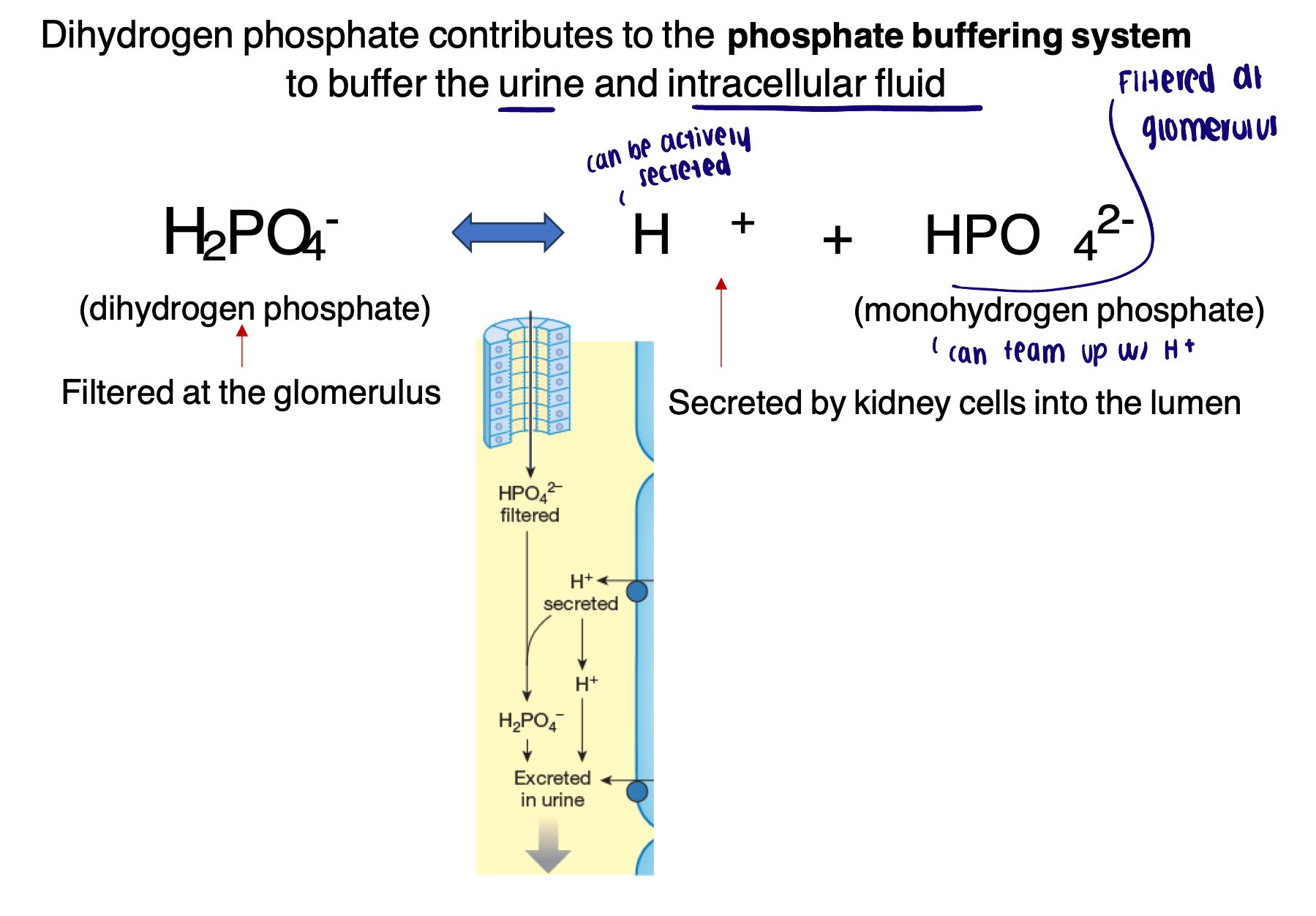
Phosphate buffer system
Dihydrogen phosphate contributes to the phosphate buffering system to buffer the urine and intracellular fluid
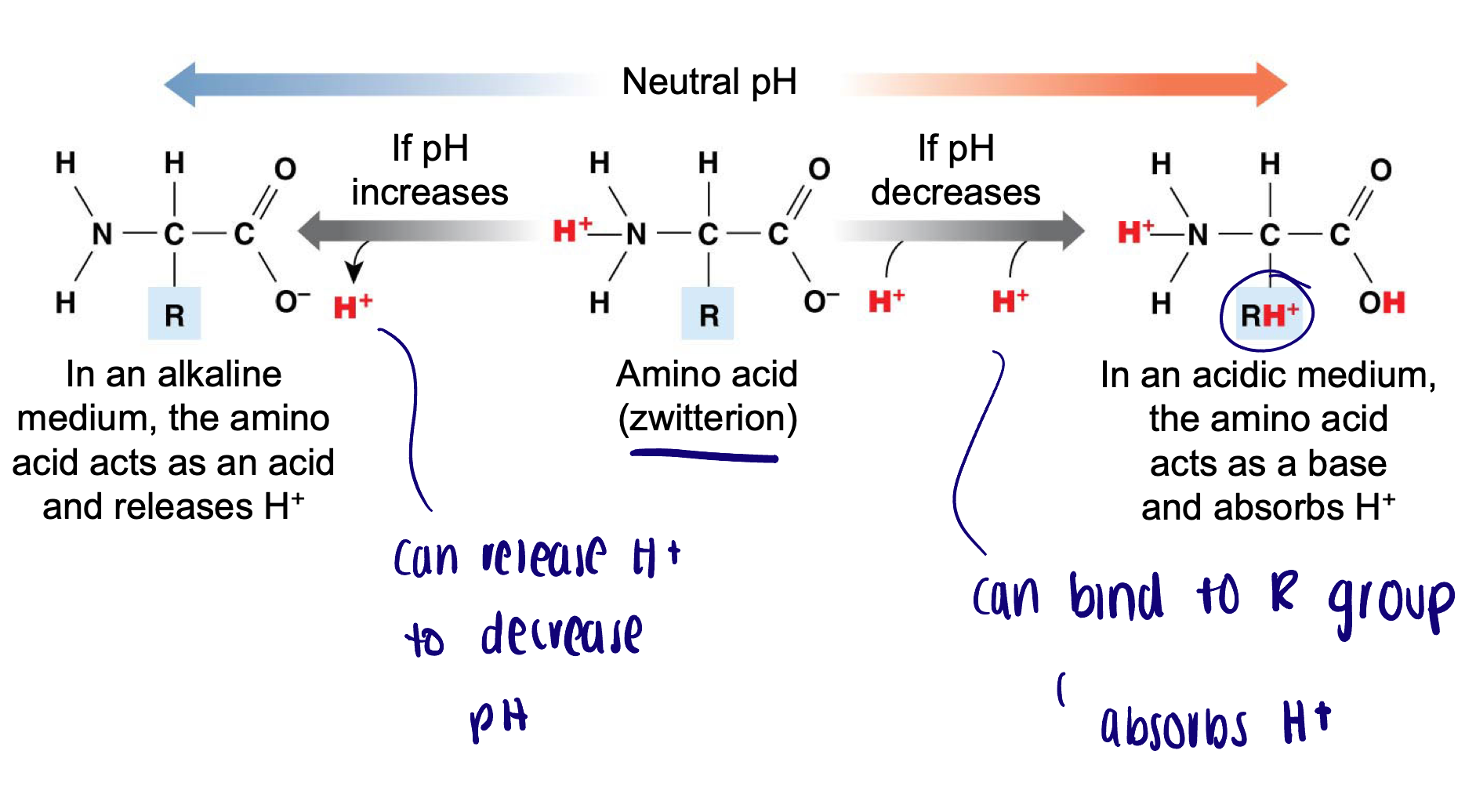
Protein buffering system
Amino acids contribute to the protein buffering system to buffer both intracellular and extracellular fluids
Amino acids are used to stabilize the pH of the cell during metabolism to prevent damage to cellular organelles
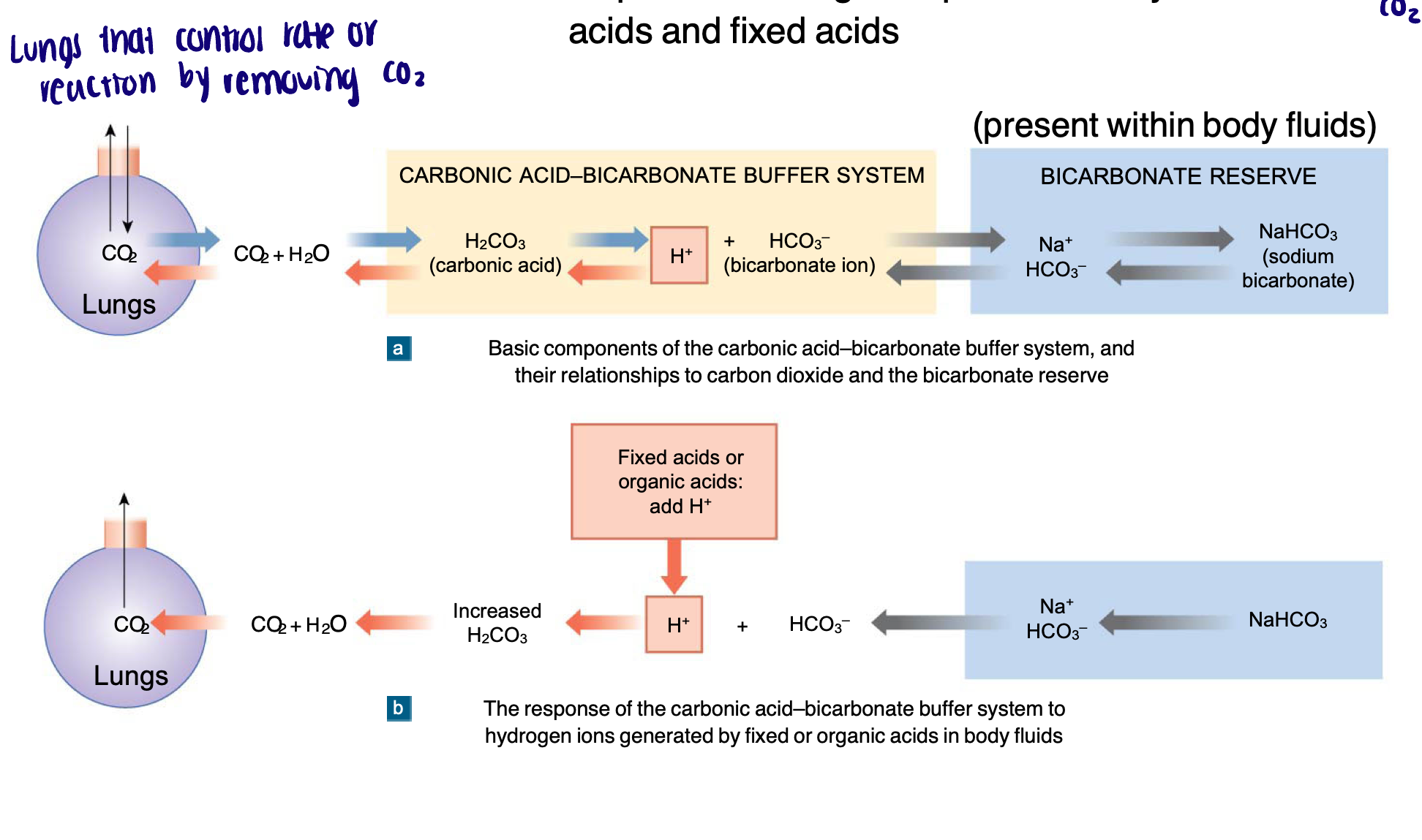
Carbonic acid-bicarbonate buffering system
Carbonic acid contributes to the carbonic acid-bicarbonate buffering system to buffer extracellular fluids to prevent changes to pH caused by metabolic acids and fixed acids
Buffers provide a __ solution to acid-base imbalance
Temporary
Ultimately, fluctuations in pH require compensation by both the lungs and the kidney
Respiratory mechanism
Carbon dioxide reacts with water to be carried in the blood
A change in rate of respiration can alter CO2 levels and drive changes in pH
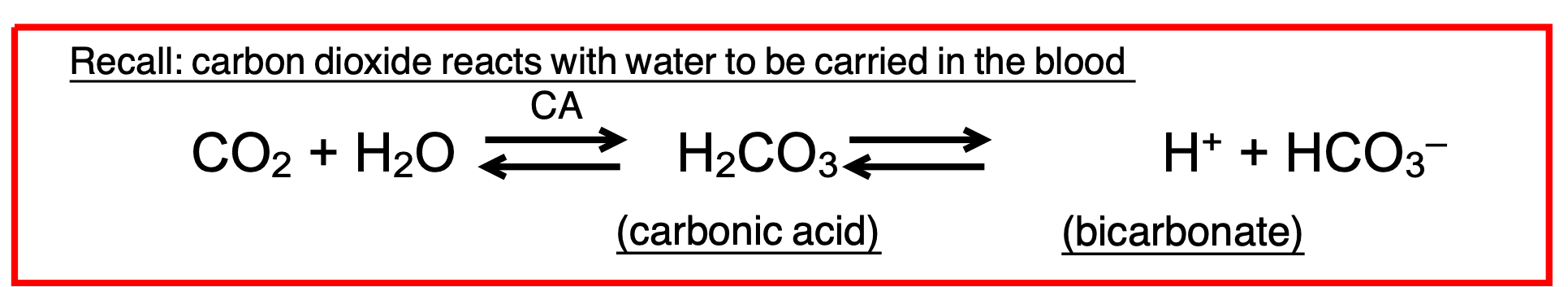
When carbon dioxide levels increase
More carbonic acid forms, additional hydrogen ions and bicarbonate ions are released, and the pH decreases
Shift equation to the right
When carbon dioxide levels decrease
The reaction runs in reverse
Carbonic acid dissociates into carbon dioxide and water
This removes H+ from the solution and increases the pH
Shift equation to the left
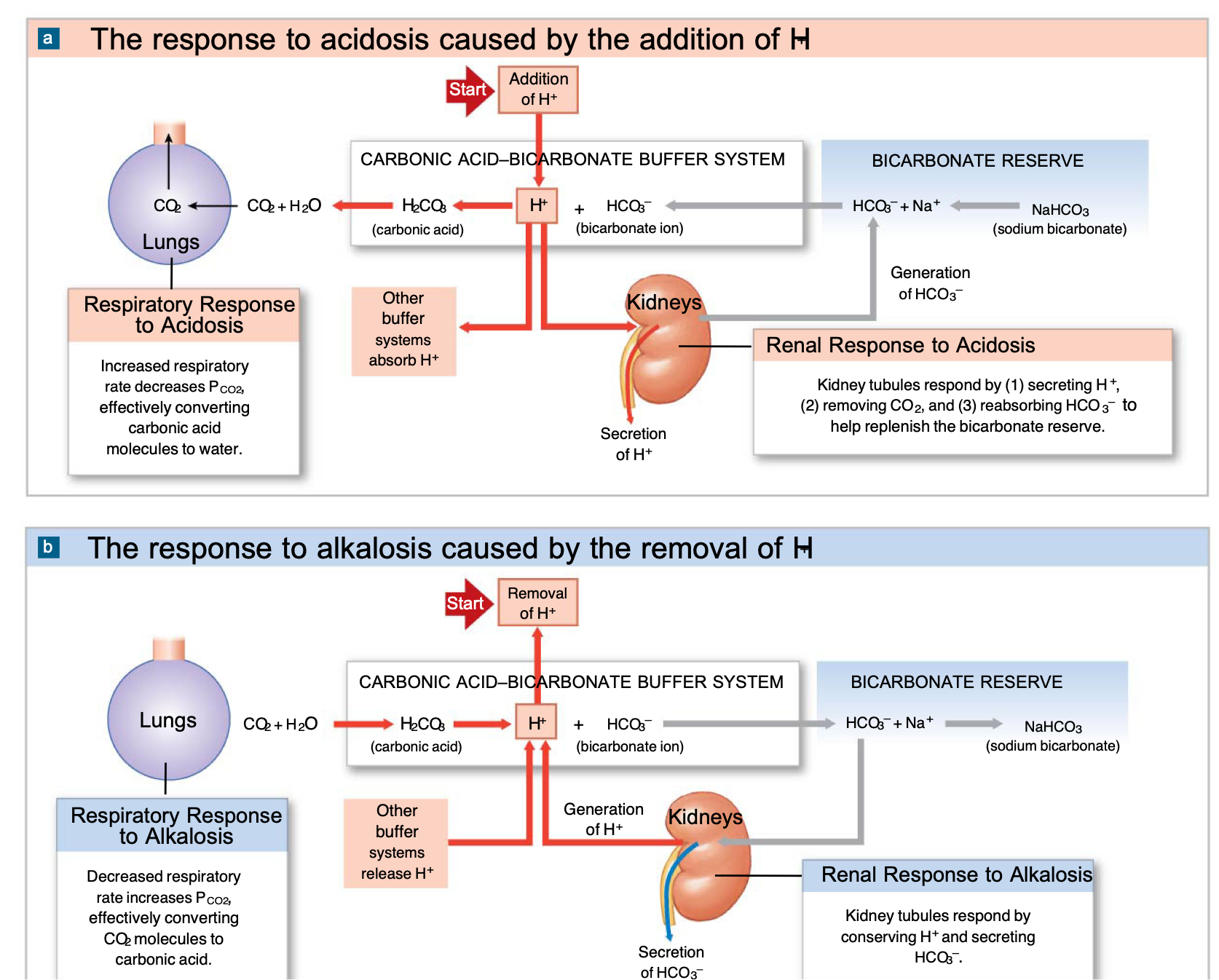
Respiratory compensation
In a state of acidosis: increase respiratory rate → decrease CO2 → increase pH (bicarbonate buffers extra H+)
In a state of alkalosis: decrease respiratory rate → increase CO2 → decrease pH (generate more carbonic acid and H+)
Renal compensation - where does H+ secretion occur and what is needed
H+ ions are secreted into the lumen in the proximal tubule, distal tubule, and collecting duct (exchanged for Na+ and Cl-)
Eliminating large quantities of H+ ions requires the presence of buffers in the lumen
Without buffers: kidneys could eliminate less than 1% of all H+ produced each day
With buffers: kidneys can maintain homeostasis by eliminating large quantities of H+ ions
All buffering systems exist within…
The kidney
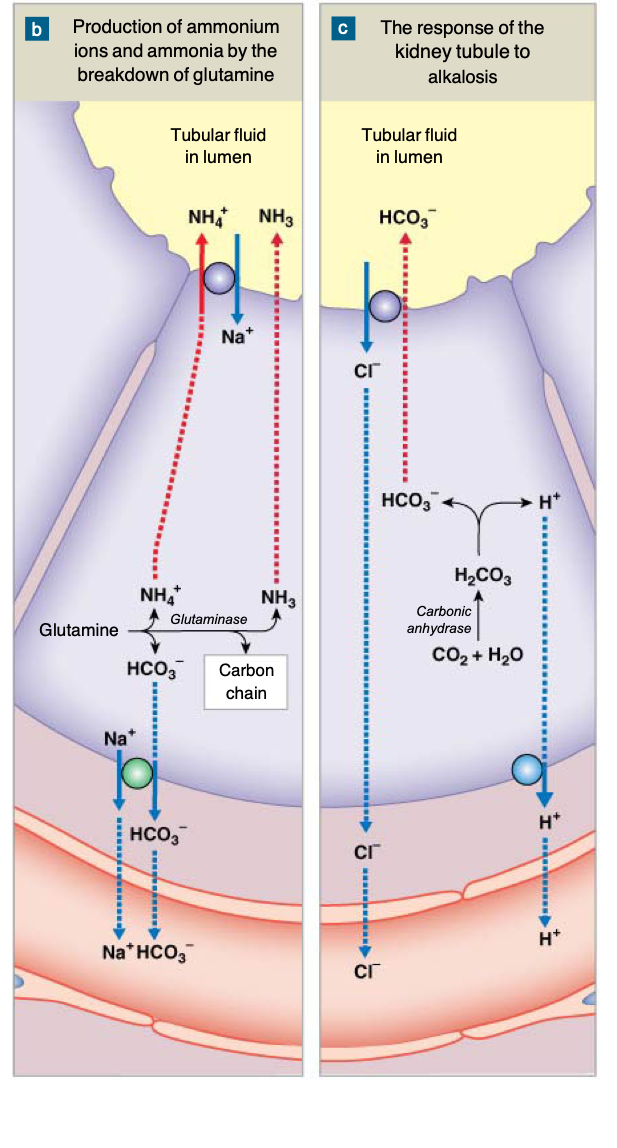
Renal compensation - ammonia buffer system
Bicarbonate and phosphate buffers are found in the renal tubule due to filtration resulting in excretion of H+
Ammonia buffer system:
The proximal tubule generates ammonia via breakdown of glutamine
Ammonia can be used to buffer H+ in the lumen
Glutamine breakdown also generates HCO3-
If acidosis: kidney increases production of ammonium ions, secretes H+, and reabsorbs bicarbonate (don’t want to lose bicarbonate in the urine)
If alkalosis: kidney conserves H+ and secretes bicarbonate
Putting everything together - diagram
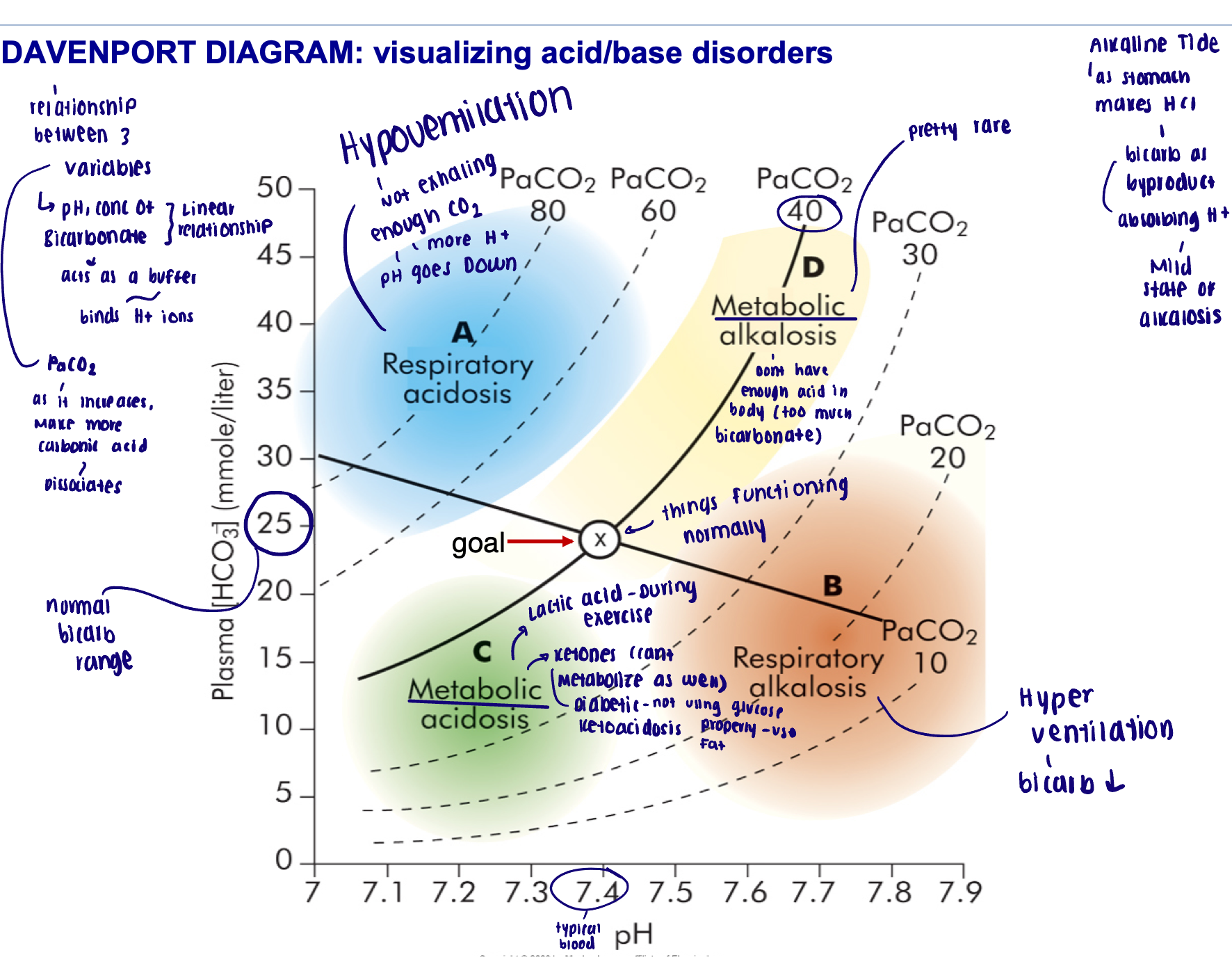
Acid/Base disorders can be…
Respiratory
Metabolic
Respiratory - acid/base disorders
Acidosis: respiratory system cannot eliminate all of the CO2 generated by the tissues
Low respiratory rate (hypoventilation)
Alkalosis: CO2 is below normal (hypocapnia)
Hyperventilation
Metabolic - acid/base disorders
Acidosis: overproduction of acid; H+ release overwhelms the buffering system
Lactic acidosis or ketoacidosis - prolonged exercise generates lactic acid buildup; starving leads to the breakdown of lipids and ketone bodies
Impaired ability to excrete H+ by the kidneys
Loss of bicarbonate ions (cannot balance the H+)
Alkalosis: elevated bicarbonate levels leading to the decrease in H+
Alkaline tide: after eating a meal, high production of HCl by the stomach leads to an increase in bicarbonate secretion into the blood
Acid/base disorder diagrams
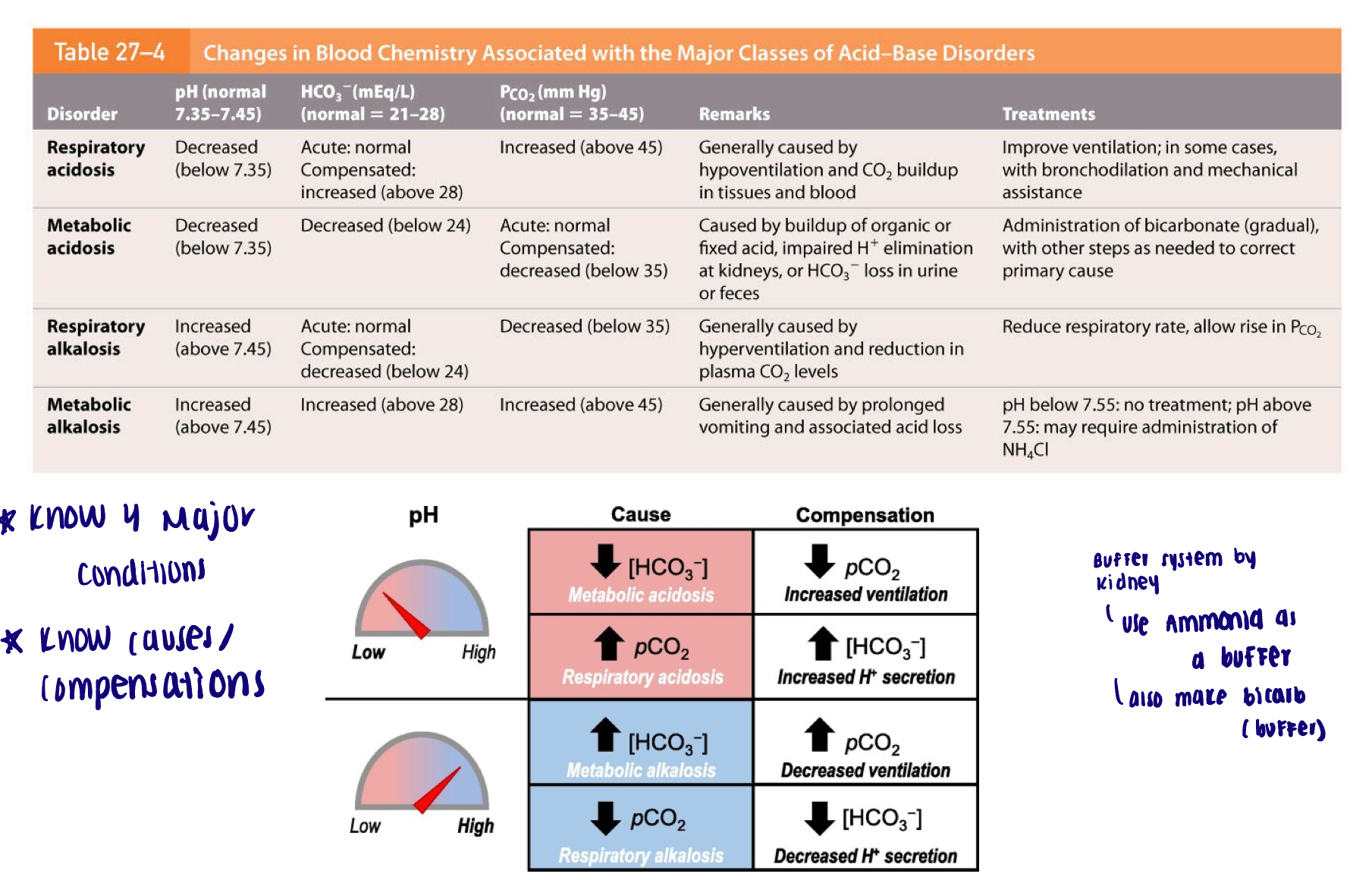
Davenport diagram
Summary of acid and bases
Normal pH
pH is regulated by…
Acidosis vs alkalosis
Respiratory compensation
Renal compensation
Maintenance of fluid pH is critical to sustain life (7.35-7.45)
pH is regulated by buffers, respiration, and urinary excretion
Acidosis → decrease in pH
Alkalosis → increase in pH
Respiratory compensation involves the control of breathing rates, changing CO2 levels
Renal compensation involves modifying H+ and CO2 excretion, ammonia production, bicarbonate reabsorption