Pharm107 - Intro to Titration
1/54
Earn XP
Description and Tags
Covering the topics of Chemical Basis of Titrimetry up to Titration Curves
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
55 Terms
Titrimetry
A group of chemical methods of quantitative analysis in which the concentration of an analyte is determined based on its stoichiometric reaction with a reagent of established concentration introduced to a sample gradually, in small portions until the analyte is consumed quantitatively
“Chemical Methods of Quantitative Analysis”
Involves chemical reactions between the analyte and a reagent.
Requires that substances are chemically reactive in the given chemical environment.
Stoichiometry in Titration
Ensures reacting species combine in fixed ratios based on their balanced chemical equation.
Example:
If 10 equivalents of NaOH are required to neutralize HCl, then the solution must contain 10 equivalents of HCl.
Equivalents: The amount of a substance that reacts in a 1:1 ratio with another.
Interfering Species in Titration
Titration accuracy depends on minimal interference from other substances.
Example:
Both Mg²⁺ and Al³⁺ react with EDTA in a 1:1 ratio.
To analyze Mg²⁺ without interference, the solution is buffered at pH 10, where Al³⁺ is masked by Triethanolamine (TEA).
This ensures only Mg²⁺ reacts with EDTA.
Standard Solution (Standard Titrant)
A reagent of known concentration used in titrations.
Also called Volumetric Solution (VS).
Normality (N) of a Solution
Expresses the number of equivalents of solute per liter (1000 mL) of solution.
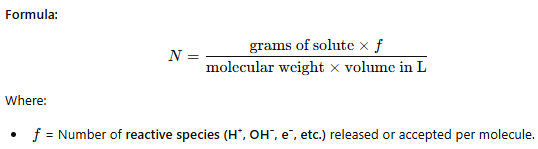
Equivalence Point
Theoretical point where titrant amount is chemically equal to the analyte.
Endpoint
Observable indication (e.g., color change) that the reaction is complete.
Appropriate Indicator
Selected with the goal of minimizing the gap between the equivalence point and the endpoint.
Indicators in Acid-Base Titration
Are weak acids/bases that change color near the equivalence point.
Examples:
Phenolphthalein: Colorless (acid) → Pink (base) at pH 8.2-10.
Methyl Orange: Red (acid) → Yellow (base) at pH 3.1-4.4.
Titration Error (Et)
The difference between the actual endpoint and theoretical equivalence point.
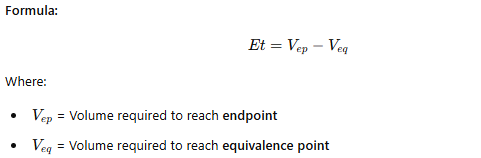
Volumetric Titration
A method wherein the volume of a standard reagent is measured and is used to quantify the unknown concentration of the analyte.
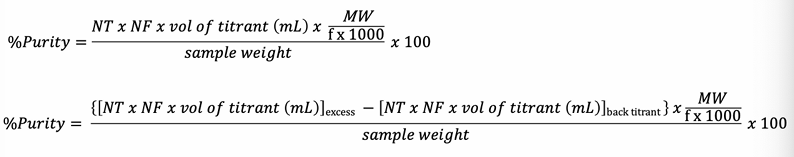
Percentage of Purity
The proportion of a pure substance within an impure sample, expressed as a percentage
Gradual Addition in Titration
Titrant must be added slowly to prevent overshooting the endpoint.
As the equivalence point approaches, titrant is added in increasingly smaller portions.
At the endpoint, even half a drop of titrant can cause a color change.
Titrant Methodology
Step 1: Titrant is added to the analyte while swirling.
Step 2: Initially, titrant is added rapidly.
Step 3: As the endpoint approaches, titrant is added in smaller portions.
Step 4: At the endpoint, even a fraction of a drop can cause a color change.
Types of Titration (Based on Methodology)
Direct Titration
Residual Titration (Back Titration)
Titration with Preliminary Treatment
Direct Titration
The most common titration method.
Requires that the reaction occurs rapidly.
If titration is added too fast:
The endpoint may overshoot the equivalence point, leading to errors.
Back Titration (Residual Titration)
Used when direct titration is impractical (e.g., slow reactions, insoluble analytes).
Steps:
Add excess titrant to react with the analyte.
Introduce a second titrant to measure the unreacted portion.
Subtract the excess titrant from the total added to determine the exact analyte concentration.
Back Titrant
The second titrant or the standard solution used to quantify the excess titrant.
Formaldehyde Analysis
An example of Back Titration wherein the reaction is slow, making direct titration difficult.
Excess iodine solution (I₂ VS) is added.
Wait 15 minutes to allow the reaction to complete.
Remaining unreacted iodine is titrated to determine the formaldehyde content.
Titration with Preliminary Treatment
Also called Indirect Titration
Used when the analyte cannot be directly titrated.
The analyte undergoes a chemical reaction or separation to form a titratable species.
Kjeldahl Titration for Urea Analysis
An example of Indirect Titration
Digestion: Urea is converted into ammonia (NH₃).
Distillation: NH₃ is separated and titrated directly.
Blank Correction
The endpoint in titration is an estimate of the true equivalence point.
Why use blank correction?
Accounts for impurities or side reactions.
Increases the accuracy of results.
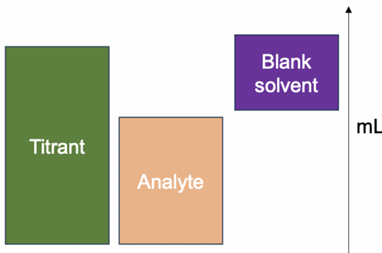
Blank Correction Methodology
Perform titration with the analyte and record the volume of titrant used.
Perform a second titration without the analyte (blank titration).
Subtract the blank titration volume from the original titration volume.
Types of Titration (Based on Chemical Reactions)
Neutralization Titration
Redox Titration
Complexometric Titration
Precipitation Titration
Neutralization Titration
Used in acid-base titrations.
The endpoint is detected using:
pH indicators (e.g., phenolphthalein, methyl orange).
pH meters for precise measurement.
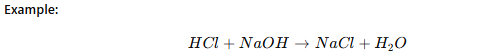
Redox Titration
Involves oxidation-reduction reactions.
One species loses electrons (oxidation), while the other gains electrons (reduction).
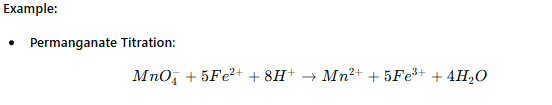
Complexometric Titration
Used for metal ion determination.
A metal ion reacts with a chelating agent (e.g., EDTA).
Example:
Determination of Ca²⁺ and Mg²⁺ using EDTA.
Precipitation Titration
The analyte forms an insoluble precipitate with the titrant.
Example:
Determination of chloride ions (Cl⁻) using silver nitrate (AgNO₃).
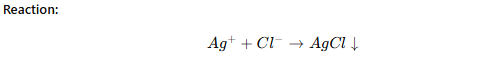
Types of Titration (Based on Detection Methods)
Visual Methods
Potentiometric Methods
Amperometric Methods
Coulometric Methods
Thermometric Methods
Automated Titrations Visual Detection Method
Visual Detection Method
Uses an indicator to signal the endpoint through a color change.
Common in acid-base titrations.
The indicator must have a clear and sharp color transition.
Examples:
Phenolphthalein: Colorless → Pink at pH 8.2-10.
Methyl Orange: Red → Yellow at pH 3.1-4.4.
Mixed Indicators
A combination of two or more indicators.
Used when a single indicator does not provide a sharp color change.
Example:
Titration of NaOH + Na₂CO₃ mixture using HCl.
Uses phenolphthalein (first endpoint) and methyl orange (second endpoint).
Potentiometric Detection Method
Measures voltage (electrical potential) changes during titration.
No indicator needed, ideal for colored or turbid solutions.
Uses indicator and reference electrodes (e.g., pH meter).
Equivalence point found at sharpest voltage change.
Amperometric Detection Method
Measures microcurrent changes as titrant is added.
Used in precipitation titrations (e.g., AgNO₃ titrations).
Requires two electrodes to monitor current flow.
Precise for low-concentration analytes.
Coulometric Detection Method
Determines analyte concentration by measuring electrical charge.
Based on Faraday’s Law of Electrolysis.
Used in Karl Fischer titration for trace water analysis.
Highly accurate for very small analyte amounts.
Thermometric Detection Method
Tracks temperature changes caused by the reaction.
Works well in acid-base titrations and exothermic reactions.
No indicator needed, useful for cloudy or colored solutions.
Endpoint found at the sharpest temperature change.
Automated Titration Method
Uses a computerized system for precise titration.
Motorized burette adds titrant at a controlled rate.
Sensors (e.g., pH meters) detect endpoint automatically.
Minimizes human error and improves reproducibility.
Standardization
Process of determining the exact concentration of a volumetric solution.
Ensures titration results are accurate and reproducible.
Uses a primary standard, secondary standard, or another standard solution.
Essential for reliable titrimetric analysis.
Volumetric Standards
Standard solutions used in titration.
Must be stable, react completely, and have a known concentration.
Used to quantify an analyte’s concentration with precision.
Commonly expressed in normality (N) or molarity (M).
Ideal Characteristics of a Volumetric Standard
Stable: Does not degrade over time.
Fast-reacting: Ensures quick, complete titration.
High purity: Minimizes errors in concentration calculations.
Well-defined reaction: Follows a balanced chemical equation.
Preparation of Volumetric Solutions (VS)
Prepared using official procedures from the pharmacopeia.
Measured with volumetric glassware for precision.
Prepared at standard temperature (25°C).
Concentration must be verified through standardization.
Empirical Concentration
The actual concentration of a solution, determined experimentally.
May differ from the theoretical concentration due to impurities.
Requires standardization to obtain accurate values.
Standardization of Volumetric Solutions
A volumetric solution's concentration is determined by titration against:
A primary standard.
A secondary standard.
A standard solution of known concentration.
Ensures the solution’s concentration is accurate and reliable.
Primary Standard
A highly pure, stable compound used for standardization.
Must have a well-defined composition and high molar mass.
Used to determine the exact concentration of secondary standards.
Example: Sodium carbonate (Na₂CO₃) for acid titration.
Characteristics of a Primary Standard
High purity: Minimal contamination.
Atmospheric stability: Does not react with air or absorb moisture.
Absence of hydrate water: Water content remains constant.
Modest cost
Reasonable solubility: Must be soluble in titration medium.
Reasonably large molar mass: Reduces weighing errors.
Secondary Standard
A compound whose concentration is determined by standardization.
Used as a working standard in titration.
Less pure and requires validation against a primary standard.
Example: Sodium hydroxide (NaOH), which absorbs moisture and CO₂.
Titer Value
The mass of a substance (in grams) that reacts with 1 mL of a standard solution.
Used to determine the exact analyte concentration.
Example: Hydrochloric acid titer value in acid-base titrations.
Normality Factor (Correction Factor)
A correction value used to adjust standard solution concentration.
Accounts for deviations from the expected normality.
Ensures greater accuracy in titration calculations.
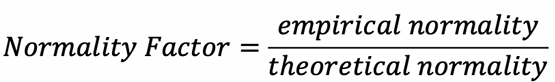
Titration Curve
A graphical representation of a titration process.
Plots p-function of the analyte (e.g., pH) vs. titrant volume.
Used to identify the equivalence point and reaction behavior
Types of Titration Curves
Sigmoidal Curve
Linear Segment Curve
Sigmoidal Titration Curve
Shows a gradual change before a sharp increase/decrease at the equivalence point.
Common in acid-base titrations.
Advantage: Fast and convenient interpretation of endpoint.
Linear Segment Titration Curve
Signal is proportional to analyte or titrant concentration.
Used when reactions require excess reagent to complete.
Example: Redox titrations with slow equilibrium.
Inflection Point
The point where the curve’s concavity changes.
Represents the steepest slope in the titration curve.
Marks the equivalence point in sigmoidal curves.
X-axis of a Titration Curve
The volume of a titrant added to the solution.
Y-axis of a Titration Curve
The pH of the overall solution.