Pharm Lecture 2
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
What are cytochrome P-450 isoenzymes?
- heme-containing isoenzymes that are involved in phase 1 reactions of metabolism
- BASICALLY: a class of enzymes whose pathways many drugs metabolize through
What are cytochrome P-450 isoenzymes named after?
named after the max light absorption wavelength (450nm)
Where are cytochrome P-450 enzymes located?
- phospholipid bilayer of the smooth ER
- ex: hepatocytes, enterocytes of small intestine, kidneys, lungs, brain
The nomenclature for a cytochrome P-450 enzyme is CYP-3A4. What does this stand for?
- CYP: cytochrome isoenzyme ("sip")
- 3: gene family (humans only have 1,2,3)
- A: gene subfamily (can be anything)
- 4: individual gene
How does a substrate pass through a CYP-450 pathway? (3 steps)
1) substrate: "lipophilic" agent (medication that needs to be metabolized)
2) metabolism of substrate by its appropriate CYP enzyme (converts lipophilic --> hydrophilic metabolites)
3) hydrophilic metabolites can be excreted by kidneys
How does an inhibitor affect substrate metabolism in a CYP pathway? (3 steps)
1) inhibitor: blocks ability of enzyme to metabolize substrate
2) increases plasma levels of substrate
3) increased potential for adverse drug effects
A patient comes into the ER and is currently taking Drug A which lowers blood pressure. You give the patient Drug B in the ER, which unknown to you, is an inhibitor of Drug A's metabolism. What would you expect to happen?
drug B will inhibit metabolism of drug A --> drug A will not be excreted as normal and will accumulate in the plasma --> will cause severe drop in BP
If two medications are substrates for a particular CYP pathway, will they have a reaction?
NO! They are substrates, so they will just be metabolized through the same pathway
What will be the effect of a non-specific inhibitor?
Will inhibit all metabolic pathways
How does an inducer affect substrate metabolism in a CYP pathway?
1) inducer speeds up metablism of substrate
2) plasma conc. of substrate will decrease
3) the substrate won't have a good effect
What are 2 ways to fix the effects of interaction btwn substrate and inducer?
1) increase dose
2) change med
A patient has been taking amlodipine for high blood pressure and their BP has normalized. They come in for a different issue and you prescribe them a new medication to be taken in addition to their normal regimen of the amlodipine. A few weeks later, the patient comes in for a check in and upon vital sign intake, their blood pressure is hypertensive again. What would you suspect has happened?
new med is an inducer of amlodipine's metabolism (amlodipine is excreted from the body quicker and unable to produce its effects --> patient isn't getting the antihypertensive effect and BP returns to hypertensive state)
What determines the interaction between a substrate and inhibitor/inducer?
The half life of the inhibitor/inducer
How does the half-life of an inhibitor/inducer alter possible drug interactions?
- short half-life: faster to steady state --> quick to see interaction
- long half-life: much slower to steady state --> will take longer to see interaction
Give an example of an inhibitor with a...
a) short half-life
b) long half-life
a) Cimetidine has half life of 2 hours; at 10 hours, will be at steady state and you will begin to see reaction
b) Amiodarone has half life of 50-100 days, may not see reaction until over a year!
Which drug is an example of a universal inhibitor?
Cimetidine
When does the maximum effect of drug-drug interaction occur? (in terms of inhibitor and a substrate)
When substrate and inhibitor achieve steady state (about 5 half lives)
Let's say patient is receiving drug A for a few years and they recently develop an arrhythmia. You prescribe amiodarone for the patient, not knowing it is an inhibitor of Drug A. When would you expect to see the drug-drug interaction?
may take up to a year to show because of the long half life of amiodarone
Give an example of an inductor with a...
a) short half-life
b) long half-life
a) Rifampin induction is apparent within 24 hrs
b) Phenobarbital induction is apparent within 5 days
What decreases a medication's induction capabilities?
1) Age (as we get older, we can no longer induce our metabolic pathways as well)
2) Hepatic disease (can lead to toxicosis)
Are elevated liver enzyme levels an indication of drug induction?
NO; liver enzymes (AST, ALT) will only show if there is hepatic disease
Knowing if the patient has hepatic disease, we can then determine the effect on induction, but it is not a direct correlation between liver enzymes and drug induction.
What is a genetic polymorphism in terms of pharmacology? What are the 2 types?
some patients are more predisposed to be poor or really good metabolizers of a particular pathway based on genetics
1) poor metabolizers
2) extensive metabolizers
Will patients need a lower or higher dose for each type of polymorphism?
a) poor metabolizers
b) extensive metabolizers
a) need a lower dose (the drugs will accumulate in their body!)
b) need a higher dose (since they excrete drugs quicker)
For poor metabolizers of CYP-2D6,
a) % of Caucasians?
b) % of African-Americans and Asians?
c) 4 examples
a) 5-10% Caucasian
b) 1-3% of African Americans and Asians
c) Donepezil, Risperidone, Haloperidol, Metoprolol
What are 6 CYP2D6 inhibitors?
1) Amiodarone
2) Cimetidine
3) Fluoxetine
4) Paroxetine
5) Sertraline
6) Quinidine
For extensive metabolizers of CYP-2D6
a) % of Caucasians?
b) % of Asians and Native Americans?
a) 90%
b) >98%
What are the statistics for genetic polymorphism of CYP-2C19?
1) 20% of African Americans and Asians are poor metabolizers
2) 3-5% of whites are poor metabolizers
Which two CYP pathways have no genetic polymorphisms?
3A4 and 1A2
What is the P-glycoprotein efflux pathway? What does it do?
- ATP-dependent transcellular transport system
- pumps toxic/foreign substances out of cells
- prevents intracellular accumulation of anticancer medications
Where is the P-glycoprotein efflux pathway located?
- on the inside of the intestinal wall: pumps drugs back out into the lumen, preventing it from reaching plasma
- located in liver, pancreas, kidneys, colon, jejunum, placenta, and brain capillaries
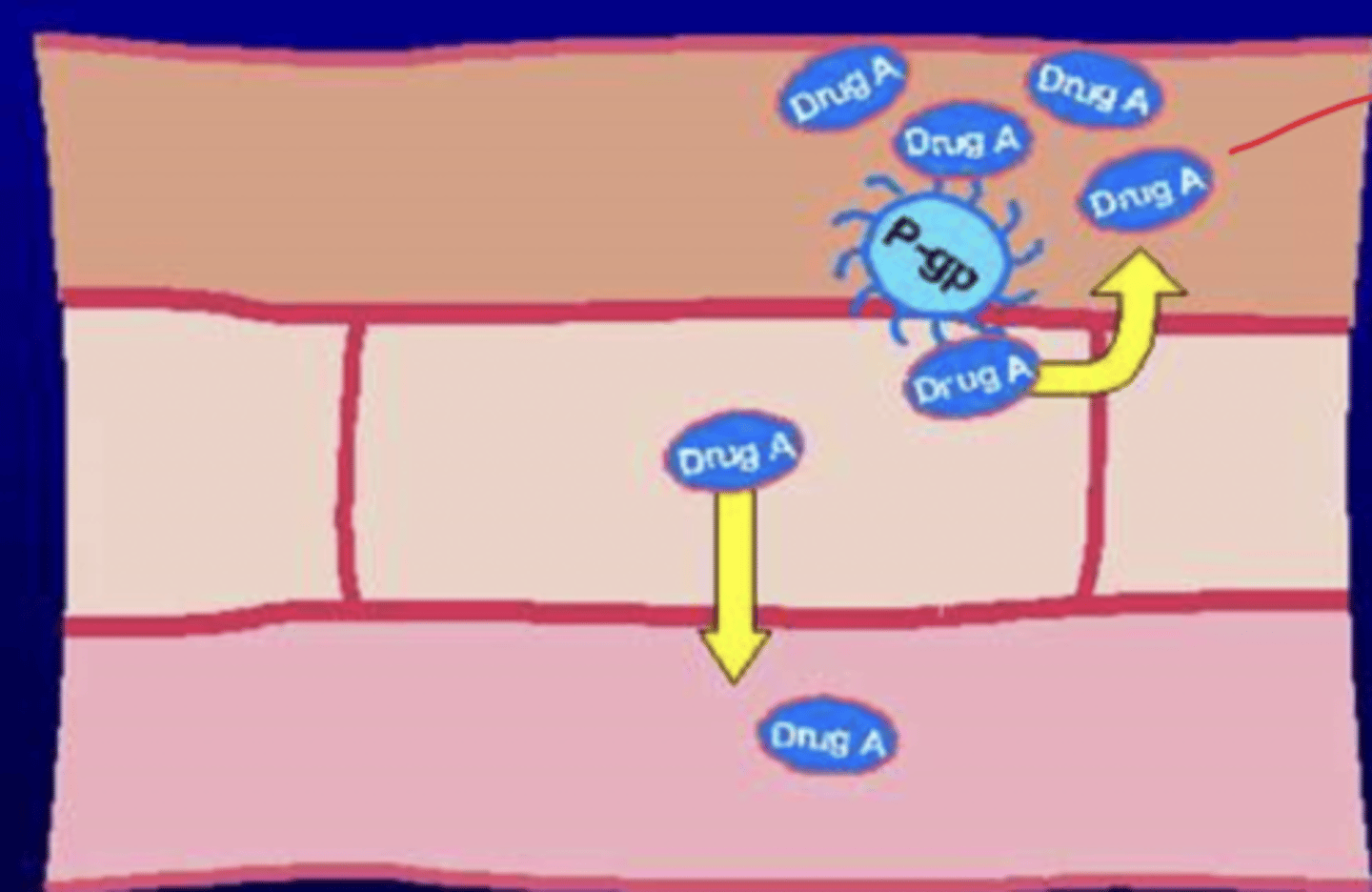
Drug A is a substrate for P-gp. How will P-gp affect the absorption of Drug A?
- drug A will reach the intestinal wall, but P-gp will pump it back into the intestinal lumen
- most of it cannot reach the plasma to enter bloodstream and travel to its target organ
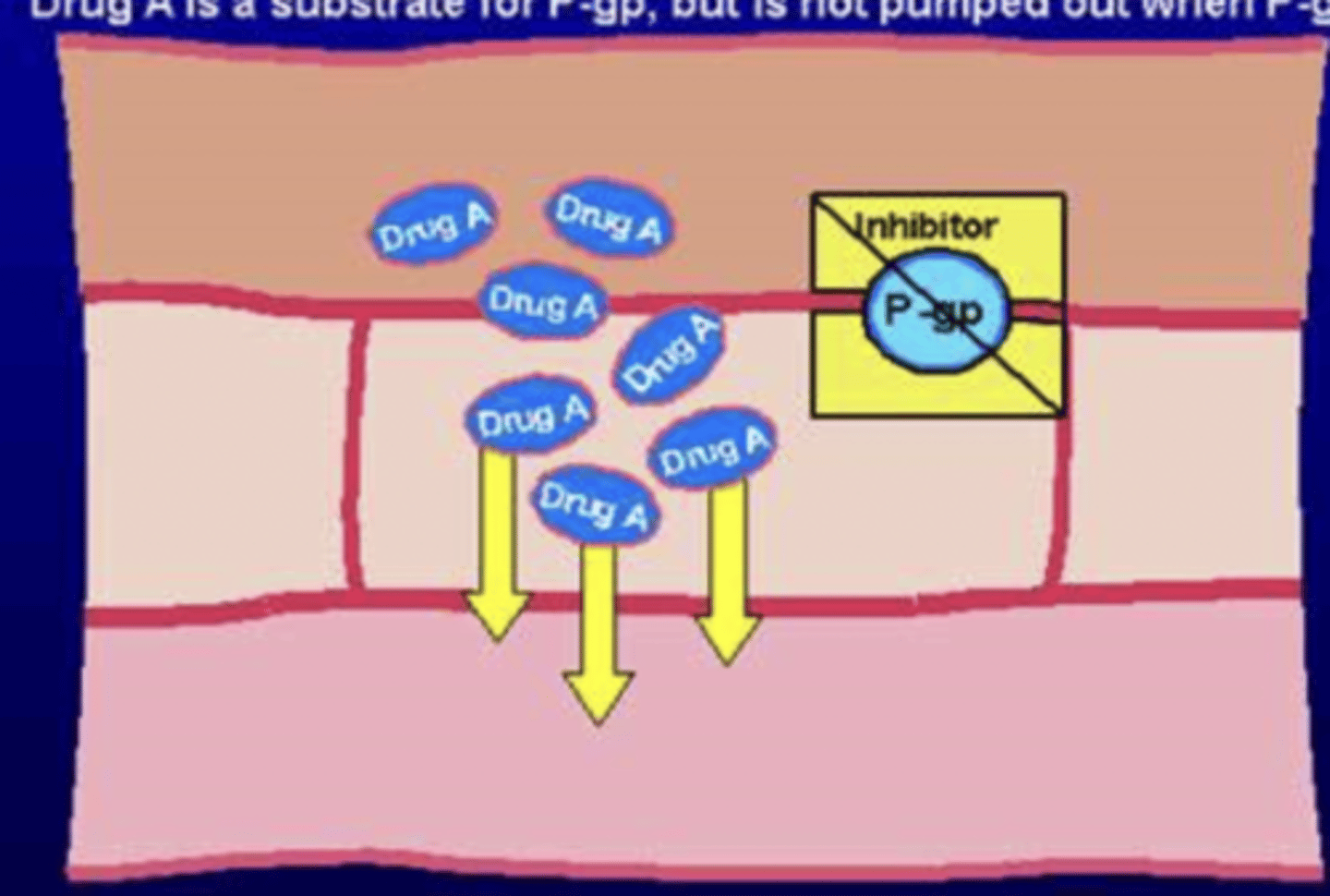
Drug A is a substrate for P-gp. What would happen if a P-gp inhibitor was added to the metabolic pathway?
- drug A will reach the intestinal wall, but is NOT pumped out bc P-gp is blocked
- nothing will bring the drug back to the lumen
- plasma concentration of A increases --> leads to toxicosis
A 55 year old was admitted to the ED for altered mental status and alcohol withdrawal. He has a history of depression treated with paroxetine 50 mg once daily and a history of HTN treated with lisinopril 20 po mg once daily and metoprolol 50 mg bid. The dose of metoprolol was increased to 100 mg po bid for better BP control. 3 hours post metoprolol, the patient became light-headed with a HR of 44 bpm. What happened?
What do we know?
- Paroxetine is known to inhibit the CYP-2D6 pathway
- Metoprolol is a known substrate of the CYP-2D6 pathway
- therefore, paroxetine would increase the concentration of metoprolol in the blood by blocking its metabolic pathway
So what happened?
- at 50 mg bid of metoprolol, the patient was tolerating the 2 meds together
- however, paroxetine + 100 mg of metoprolol = too much metoprolol in plasma
- since metoprolol decreases BP --> drastic drop in BP which led to light-headedness and bradycardia
What kind of pathway does digoxin operate under?
substrate for P-gp pathway
AA is a 65 year old male on maintenance therapy with Digoxin for mild congestive heart failure. He receives a 2nd prescription for fluconazole for the management of a fungal infection. Shortly after he begins taking the fluconazole, he experiences 3rd degree heart block. Lab studies indicate increased plasma concentrations of digoxin. What happened?
What do we know?
- digoxin is a substrate for P-gp
- digoxin also has a narrow therapeutic index
So what happened?
- fluconazole was a Pgp substrate or inhibitor, which led to increased digoxin plasma concentration and became toxicity
What are 7 CYP-3A4 inducers?
1) Carbamazepine
2) Phenytoin
3) Phenobarbital
4) Rifabutin
5) Rifampin
6) Primidone
^universal inducers
7) Hypericum (St.John's Wort)
What is St. John's Wort?
- an OTC supplement taken for anti-depressive effects
- hypericum (active ingredient) is very similar to serotonin, can boost serotonin plasma levels
Why should St. John's wort not be taken with SSRI's (Prozac, Zoloft, etc.)?
- these drugs also increase serotonin plasma levels
- can lead to serotonin syndrome --> presents with fever, increased hart rate, blurry vision
In summary, what are 2 roles of St.John's Wort in drug interactions?
1) additive: leads to serotonin syndrome with SSRIs
2) inducer: can cause meds that get metabolized via CYP-3A4 to increase in plasma --> toxicity
What are 3 pharmacodynamic interactions?
1) additive (ex: St.John augments SSRIs)
2) synergistic (2 drugs work together for a better effect)
3) antagonistic (1 drug blocks the effect of another)
What are 4 pharmacokinetic interactions?
1) altered drug absoprtion
2) altered drug distribution
3) altered drug biotransformation (metabolism)
4) altered drug excretion (elimination)
How does grapefruit interact with drugs?
- all forms of grapefruit juice inhibit CYP3A4
- interaction lasts for 4-7 days, so spacing won't work
What is treatment duplication?
- a person may end up taking more than the therapeutic dose of a medication by accidentally taking two meds that contain the active ingredient
- ex: acetaminophen + OTC cough/cold medication
What is an example of antagonism in drug-drug interactions?
- NSAIDs and antihypertensive agents
- antihypertensive agents: increase salt/water excretion by kidneys
- NSAIDs: restrict blood flow to kidneys, increases BP
- result: destruction of kidney; hypertensive agents aren't as effective
What is chelation?
- binding of a drug to ions
- prevents free form of medication from reacting with the body
Which 3 antibiotics should you avoid taking with milk, calcium, aluminum, and magnesium containing products? Why?
1) Fluoroquinolones
2) Tetracyclines
3) Doxycyclines
- all 3 undergo chelation process
- ex: the Ca2+ in milk can bind to tetracycline, reducing the effectiveness of the antibiotic
How is chelation indicated in antibiotic resistance?
If an antibiotic is chelated and not absorbed properly it can lead to abx resistance
What is polypharmacy? In what population is it typically seen?
- the use of more medication than is clinically indicated or warranted
- ex: 5+, 7+ drugs
- typically seen in the elderly population (about 55-59%)
What usually causes polypharmacy?
a "prescribing cascade" or "treating" an adverse effect of one drug with another
ex: 65 y/o female admitted for CAP is started on Levaquin and develops a rash --> is given Benadryl for the rash, which causes constipation --> is given Colace for the constipation, which causes diarrhea --> diarrhea causes electrolyte imbalances and hypokalemia --> is transferred to the critical care unit and given Flagyl (preventative for C. diff) --> gets nausea from the Flagyl --> is given Reglan for the nausea which gives her tremors... etc.
How can we decrease polypharmacy?
Discontinuing unnecessary medications!!
What is the Joint Commission?
- the national accrediting body of health care organizations, regulates hospitals and publishes rules/procedures that they must abide by
- allows institutions to be accredited which permits them to get reimbursed for Medicare/Medicaid
What is the standard given by the Joint Commission for medication orders?
must be clear and accurate
What are the elements of performance set in place by the Joint Commission?
- for every medication, there is a documented diagnosis, condition, or indication for use
- ex: Tylenol PRN, must have documentation why the patient is on Tylenol so another provider can evaluate if it is still needed
What does polypharmacy lead to? (5)
- More adverse drug reactions
- Decreased adherence to drug regimens
- Drug-drug interactions (and drug toxicities)
- Poor quality of life
- "Unnecessary" drug expenses
What is the most consistent risk factor for adverse drug reactions?
Number of drugs being taken
Risk exponentially increases as the number of drugs increases
How does aging affect absorption of drugs? (2)
GI changes:
1) Decreased gastric acidity (increase in pH)
2) Decreased GI motility and delay in gastric emptying
How does decreased gastric acidity in elderly population affects absorption of some drugs?
- some medications need sufficient acidity in order to be absorbed (for example, Ca2+)
- decreased gastric acidity decreases absorption of calcium carbonate and iron salts
What can elderly patients do in order to absorb calcium? (3)
1) take calcium citrate (better alternative)
2) take calcium carbonate w/orange juice or vit C for better absorption
3) lots of elderly patients take Ca2+ supplements to prevent osteoporosis!
How does decreased gastric motility and delay in gastric emptying in elderly populations affects absorption of drugs? (2)
1) esophageal transit time is increased --> can lead to esophageal injury (ex: doxycycline, iron sulfate, potassium chloride, sitting upright after taking alendronate)
2) increased risk for ulcerations (ex: aspirin, NSAIDS)
How does aging affect distribution of drugs? (3)
1) decrease in total body water + increase in total body fat
2) decrease in VD for water-soluble drugs (ex: digoxin, lithium, theophylline) + increase in VD for lipophilic drugs
3) decrease in plasma albumin levels by 15-20%
What is the clinical effect of lower plasma albumin levels in elderly populations?
lower plasma albumin levels --> increase in free fraction of medications in the plasma (not enough albumin to bind the medication) --> can lead to toxicity
What is the Shiner-Tozer equation?
Estimates the corrected serum concentration of the anti-seizure drug phenytoin in patients with low albumin levels (hypoalbuminemia)
Ccorrected = (Cobserved) / 0.2(albumin) + 0.1
In renal failure where QCr is less than 15 mL/min:
Ccorrected = (Cobserved) / 0.1(albumin) + 0.1
What is QCr?
- also written as CrCl
- creatinine clearance
A 68 y/o female nursing home patient was admitted to the ICU for severe pneumonia and respiratory failure. The patient has a history of seizures, treated with phenytoin (loves to bind to albumin) 300 mg once daily.
CrCl = 35 mL/min; albumin levels = 1.5
Day 1:
- Phenytoin conc. upon admission = 8 mcg/mL (lower than avg which is 10-20 mcg/mL)
- The patient receives 2 boluses of 200 mg each (provider wanted to bump up the concentration!)
Day 2:
- Post loading dose, phenytoin concentration = 17 mcg/mL
- Patient is lethargic and not responsive
- No other medications known to cause CNS depression
What happened?
Phenytoin follows zero order kinetics --> increase in dose does not mean equal increase in plasma concentration
Albumin levels are low (typically 3.5-5), so pt is hypoalbuminemic --> need to use the Shiner-Tozer equation to determine the amount of free-form phenytoin in the body
Initially: Ccorr = 8 / (0.2)(1.5) + 0.1 = 20 mcg/mL (in the normal range!)
After: Ccorr = 17 / (0.2)(1.5) + 0.1 = 40 mcg/mL (way too high and led to her symptoms)
What toxicity can each phenytoin concentration lead to?
a) >20 mcg/mL
b) >30 mcg/mL
c) >40 mcg/mL
d) >50 mcg/mL
a) nystagmus, diplopia
b) ataxia, slurred speech, GI
c) lethargy, confusion
d) rarely choreathetoid movements + opisthotonic posturing
How does aging affect metabolism of drugs?
Decreased liver mass and hepatic blood flow --> highly variable metabolism and no good way of estimating
How does metabolism change in the elderly for...
a) phase I (P-450)
b) phase II
a) decline in phase I metabolism (oxidation, reduction, hydrolysis)
b) no change in phase II metabolism (glucuronidation, sulfation, acetylation)
What drug goes through hydrolysis and will metabolize differently in the elderly?
Diazepam has decreased drug clearance and increased half-life, leading to increased sedation bc elderly pt can't metabolize it
What are the 3 drugs that go through acetylation (phase II metabolism)?
1) Lorazepam
2) Oxazepam
3) Temazepam
mnemonic: LOT
How does aging affect excretion of certain drugs? (2)
1) Decreased renal blood flow and GFR (accumulation of renally eliminated drugs)
2) Decreased lean body mass leads to decreased creatinine production (QCr decreases by 10 mL/min per decade of lfie after age 30)
What are some examples of renally eliminated drugs that will accumulate in the elderly?
- AG, vancomycin, digoxin, lithium, ACEIs
- meperidine, normeperidine, morphine
What is the Cockcroft-Gault equation?
Estimates the QCr using the serum creatinine (SR), age, and ideal body weight (IBW)
QCr = (140-age) IBW / SrCr72
SrCr is in mg/dL and IBW is in kg
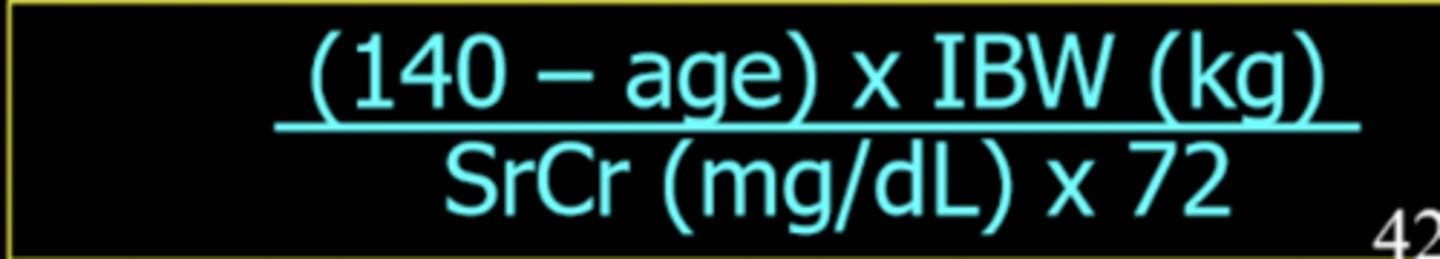
How do you alter the Cockcroft-Gault equation for women?
Multiply it by 0.85
What are 2 approximations (though not super accurate) you can make in the Cockcroft-Gault equation?
1) Using IBW instead of adjusted BW in obese patients (> 40% obesity)
2) Small elderly women with SrCr < 1 mg/dL can just use SrCr = 1 to adjust for decreased mass
How does dosage depend on the CrCl of kidney-excreted drugs?
generally, the lower the CrCl, the lower the dosage
ex) for Digoxin:
If CrCl > 50 mL/min --> taken daily
If CrCl is 10-50 mL/min --> 25-75% taken every 36 hours (need to give enough time for body to clear medication)
What are 2 other drugs whose doses can be adjusted depending on CrCl?
1) Captopril (alternative: fosinopril)
2) Levofloxacin (alternative: moxifloxacin)
What is compensatory elimination?
- bypasses dose adjustment by using the other organ of excretion
- ex: if kidneys shut down, use a drug that's metabolized via the liver (and vice versa)
What is an exception to the rule that if kidney function is impaired a non-renally excreted drug should be given?
Levofloxacin --> antibiotic for a UTI, will still need to be given because it concentrates in the urine and even if kidney function is impaired, you want to make sure the antibiotic gets to its target (urine)
A 65 y/o female (52kg; SrCr = 1.8; CrCl = 31 mL/min) is admitted to the ICU for severe sepsis. She is started on Enoxaparin (anticoagulant) 40 mg SC daily for deep venous thrombosis prophylaxis. After the third dose, the patient developed frank hematuria with slight decreased Hg/Hct. What should have been done?
Enoxaparin dosage is altered if CrCl is < or = to 30 mL/min
Even though this pt's CrCl=31, she's elderly, has low body weight, and CrCl levels are on the border, so we should follow the adjusted dose (better safe than sorry)
Reduce dose by ~ 30%
How does aging affect pharmacodynamics?
generally, lower drug doses are required to achieve the same effect with advancing age
What is inappropriate medication use?
those drugs whose use should be avoided bc their risk outweighs potential benefits
What is Beer's criteria?
- lists medications that should be avoided in the elderly
- based on specific co-morbidities in the elderly population
- includes other drugs for which doses, frequencies or duration of use should not be exceeded
What are 5 drugs listed on Beer's criteria?
1) anticholinergics
2) TCA (antidepressants)
3) 1st and 2nd gen antipsychotics
4) barbiturates (sedation)
5) long acting BDZ
For anticholinergics,
a) why should they be avoided
b) when can they be used
a) arrhythmias, dry mouth, urinary retention
b) avoid if possible
For TCA (antidepressants),
a) why should they be avoided
b) when can they be used
a) anticholinergic, orthostatic hypotension
b) a low dose can be given for neuropathic pain
For 1st and 2nd gen antipsychotics,
a) why should they be avoided
b) when can they be used
a) anticholinergic, tardive dyskinesia
b) if IV/IM medication is required
For barbiturates (sedation),
a) why should they be avoided
b) when can they be used
a) respiratory depression, habituation, risk of falls
b) use in pts with seizures
For long acting BDZ,
a) why should they be avoided
b) when can they be used
a) can lead to falls/hip fractures
b) avoid if possible
What are high-alert medications?
Medications that bear a heightened risk of causing significant patient harm when they are used in error
What are some procedures in place in regards to high-alert medications? (5)
- Hospitals each have policies and procedures
- Hospital staff education
- Close patient monitoring
- Standard patient concentrations
- Automatic stop orders: clinicians can't presribe something for more than a few days --> need to reevaluate if med is really needed when represcribing
What is the automatic stop order for narcs and anticoagulants?
3 days
What are the preferred formulations of medications for patients with tube feeding?
- solutions or soluble tablets
- some liquid formulations may not be suitable (can clog tube)
How can you avoid obstruction of feeding tubes by medications? (5)
- shake suspension well before administration
- use bulk forming laxatives
- use alternatives or dissolve w/large amount of solution
- flush before and after drug administration
- withhold tube feeding 2 hours before and after drug administration
What are 2 types of interactions that could occur with enteral feeding formulas?
1) formation of precipitation: clogs feeding tubes
2) binding to the formula content: decreases drug absorption and efficacy