Innate Immunity
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
what are some key components of the innate immune system
epithelial barriers
phagocytes
neutrophils, monocytes, macrophages, dendritic cells
type II immunity
mast cells, eosinophils, basophils
lymphoid cells
ILC1-3 & NK cells
innate T cells
NKT cells, MAIT cells, γδ T cells
complement
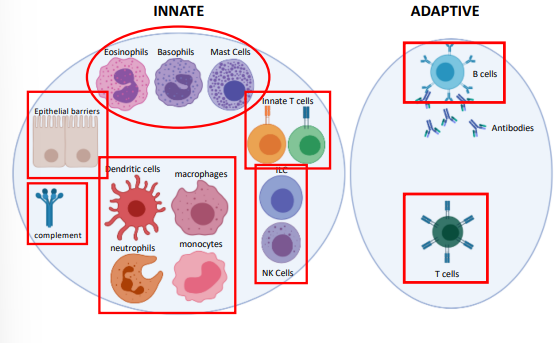
what are some components of the adaptive immune system
T cells
B cells
antibodies
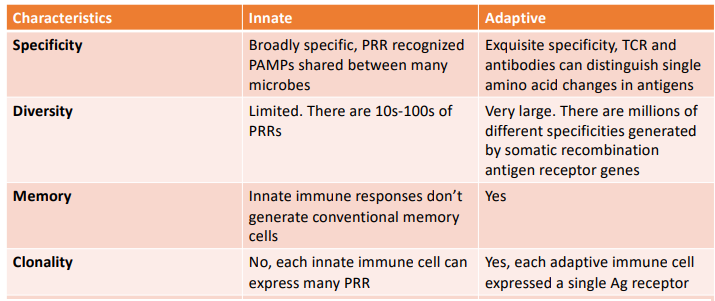
how do the innate & adaptive system respond
innate - response is rapid, can be detected within quickly
adaptive - response is slower, detected after several days
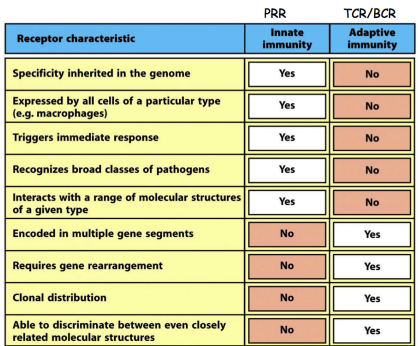
why is the innate immune system so good a detection
innate immune cells detect the expression of many different types of pattern recognition receptors (PRR)
these recognize structures of microbes not expressed in mammals
what are pattern associated molecular patterns (PAMPS)
structures that are present on microbes but not present in mammals
these are recognised by immune cells
why is the adaptive immune system so selective
each lymphocyte displays an antigen receptor with a unique single specificity
what is important to note about PRR (regarding germline)
they are germline encoded & broadly specific
cannot distinguish PAMPS from different species
EXAMPLE: can recognize LPS cant distinguish e. coli vs salmonella
what is somatic recombination
the process by which DNA segments are rearranged (V, D, J cut & rejoined) to create new combination
gives rise to receptor diversity
summary of innate & adaptive immunity
how are epithelial cells involved in an immune response
they secrete chemokines & cytokines at the basal surface that recruit immune cells
describe mucociliary transport
refers to the movement of mucus (produced by goblet cells)
usually to remove microbes from the body (e.g. lungs)
what is meant by complement
a series of serum proteins (C1-C9) that collectively form a biochemical pathway, has 3 immunological outcomes:
inflammation
opsonisation
microbial lysis
what are the 3 ways of activating complement
the alternative pathway - an ancient non-specific chemical reaction
the classical pathway - activated by complexes of antibody with antigen
The lectin pathway - triggered by recognition of unique microbial carbohydrates
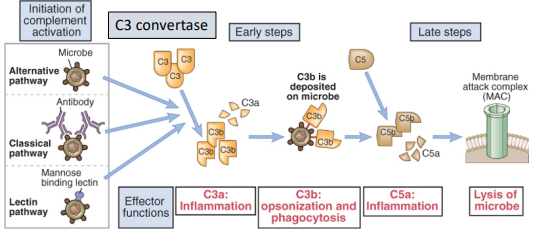
what is important to note about all 3 pathways
all pathways create a C3 convertase, after which the pathways converge
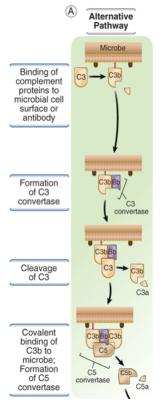
describe the mechanism of the alternative pathway
C3 spontaneously hydrolyses to from C3a & C3b
C3b then forms a C3 convertase
triggers rest of pathway
this is non-specific with no specific recognition event
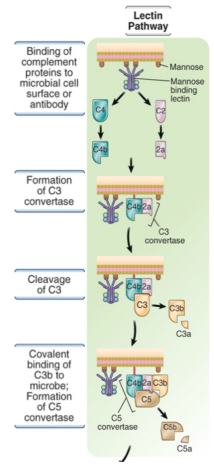
what is the mannose binding lectin
a soluble pattern recognition receptor found in serum that binds to mannose found in the surface of microbes
describe the mechanisms of the lectin pathway
proteases associated with MBL then cleave C4
rest of pathway is identical to classical pathway
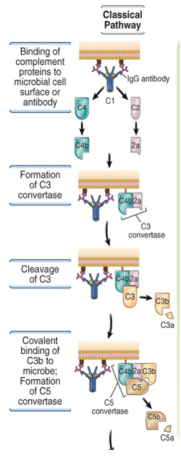
describe the mechanism of the classical pathway
C1q recognises IgG or IgM bound to antigen
C1q also contains proteases that cleave C4
C2 is cleaved generating the C3 convertase
C3b converts the C3 convertase into a C5 convertase
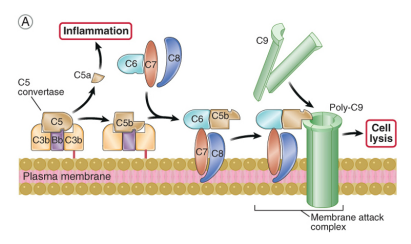
describe late stages of complement activation (all pathways)
C5 gets cleaved into C5a & C5b
C5a is a potent pro-inflammatory factor
C5b is stuck on the cell/microbe surface and initiates the assembly of the MAC (C5b, C6, C7, C8, C9)
MAC generates pores in membrane leading to cell lysis by osmotic shock
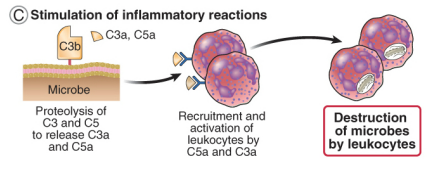
how do complements drive inflammation & leucocyte recruitment
the proteolysis of C3 & C5a release the soluble fragments C3a & C5a
leucocytes have receptors for C3a & C5a
C3a & C5a are chemo-attractants (attract leucocytes to the site of infection)
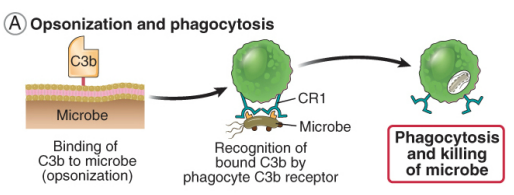
how do complements drive opsonization & phagocytosis
all complement pathways deposit C3b on the pathogen surface
phagocytes have receptors for C3b
A microbe covered in C3b is then recognized by the complement receptor which activates the phagocyte leading to phagocytosis
what is some general information on macrophages
antigen presenting cell
can differentiate into several different forms
inflammatory macrophages
anti-inflammatory macrophages
what is the function of monocytes
an emergency source of macrophages
upon infection, monocytes leave the circulation & differentiate into macrophages
arrive slower than neutrophils
what is some information about neutrophils
rapidly migrate out of circulation to site of infection
can’t present antigen, limited cytokine secretion
effective killers of organisms growing extracellular
what are the 3 mechanisms by which neutrophils can kill
phagocytosis
degranulation: release granules that contain anti-bacterial proteins
neutrophils extracellular traps (NETs), release a DNA web that traps & destroys bacteria
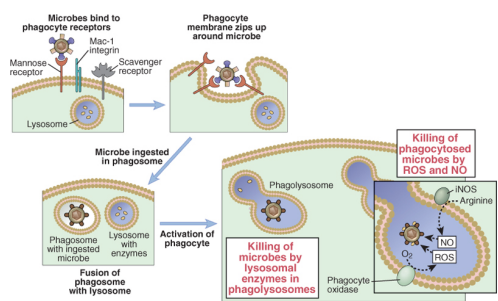
describe the process of phagocytosis
PRR specialised in uptake bind to microbes
involution of the phagocyte membrane leads to microbial uptake into the phagosome
The phagosome fuses with the lysosome – an acidic vesicle rich in degradative enzymes
In the phagolysosome two enzymes play an important role in killing the microbes:
Phagocyte oxidase - Reactive Oxygen Species (ROS)
Inducible Nitric Oxide synthase - Nitric Oxide
differences PRR vs TCR/BCR
what are the 4 major classes of pattern recognition receptors
toll-like receptors (TLR)
rig-like helicases (RLH)
nod-like receptors (NLR)
C type lectin receptors (CLR)
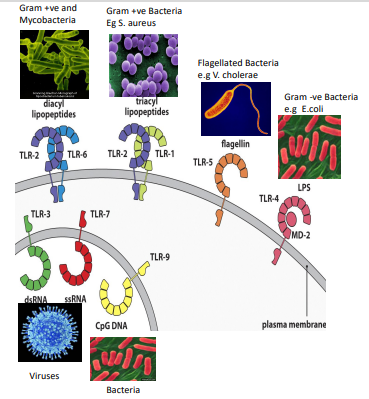
what are toll-like receptors
membrane proteins expressed either at the cell surface or in endosomes
recognize PAMPs from pathogens
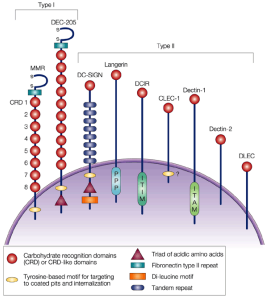
what are C-type lectin receptors
a family of membrane proteins expressed at the cell surface
many recognize polysaccharides expressed by pathogens
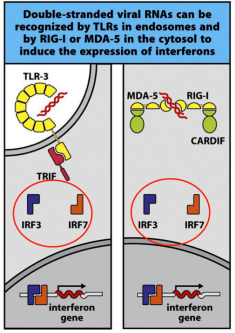
what are rig-like helicases
RIG-1 & MDA-5 are located in the cytoplasm and recognize viral dsRNA
what are nod-like receptors
a family of cytoplasmic receptors that recognize a variety of microbial products
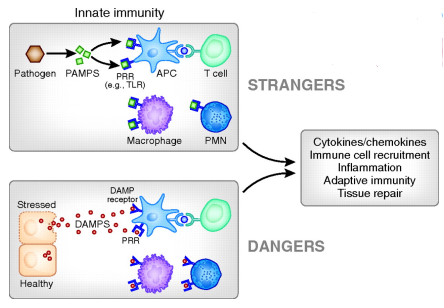
what other types of molecules can PRR recognize
endogenous ligands released upon cellular damage/stress
Damage Associated Molecular Patterns (DAMPs)
what are the 5 parts of the inflammatory response
heat
redness
swelling
pain
loss of function
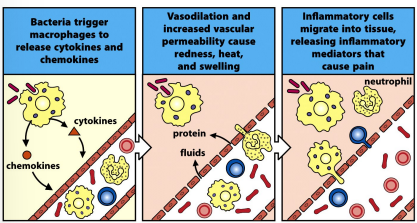
describe the general inflammatory response
tissue resident macrophages are activated via pattern recognition
release pro-inflammatory cytokines & chemokines
cytokines activate the vascular endothelium, thus vasodilation & increase permeability
redness, heat & swelling
chemokines recruit leucocytes to the site of infection, release inflammatory mediators
pain, loss of function
what is the inflammasome
a multimeric complex compromised of:
a NLR (nod-like receptor)
ASC (adaptor protein)
protease caspase 1
structure forms after the NLR binds it ligand
what are the 2 signals for the inflammasome to cleave & activate IL-1B
pattern recognize from a PRR that leads to transcription & translation of Pro-IL-1B
cleavage of Pro-IL-1B by the inflammasome
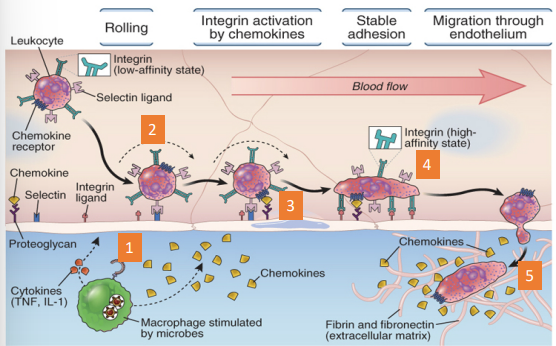
what are the different stages of leucocyte recruitment
cytokines: activate the vascular endothelium
selectins: adhesion molecules that allow leucocytes
chemokines: activate integrins to become high affinity
integrins: adhesion molecules that allow leucocytes to stably adhere
Leucocytes transmigrate out of the vasculature and follow a chemotactic gradient towards the site of infection
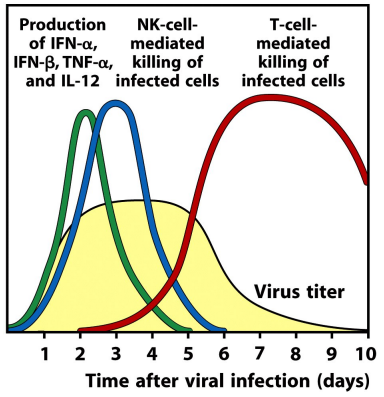
what are type I interferons & functions
cytokines with potent antiviral activity, signaling through the type I IFN receptor puts the cell into an antiviral state
inhibits viral protein synthesis
degrades viral RNA
inhibits viral gene expression & virion assembly
what other immune cells can type I IFNs activate
NK cells
dendritic cells
what are NK cells
a type of innate lymphoid cell, they kill virally infected cells
no antigen receptor
activated by type I interferon
what are dendritic cells
a type of leucocyte, role is to activate naive T cells
only antigen-presenting cell that activate naive T cells and initiates an immune response
what is the function of the T cell receptor
to recognize peptide antigens presented on MHC markers
what are the 2 types of MHC marker
MHCI - expressed by all nucleated cells
MHCII - expressed only by certain immune cells
professional antigen presenting cells
what are the 2 types of T lymphocytes
CD4+ T cells = express CD4
CD8+ T cells = express CD8
what is CD4
a cell surface molecule that can bind, in a peptide independent manner, to MHCII
when activated, CD4 T cells → T helper effector cells
what is CD8
a cell surface molecule that can bind to MHCI in a peptide independent manner
when activated, CD8T cells → cytotoxic T cells
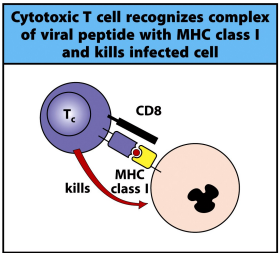
what 3 signals do naive T cells require from professional antigen presenting cells
antigen + MHC
costimulation (CD80 & CD86)
cytokines to differentiate T cells into effector cells
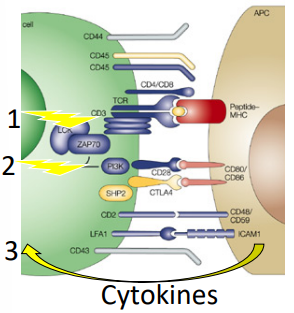
what is signal 2 (costimulation)
DC display costimulatory molecules CD80 & CD86
these interact with T cell molecule CD28
generates signal that synergizes with MHC+peptide signal
leads to T cell proliferation
what is signal 3 (cytokine production)
dendritic cells secrete cytokines that drive T cell differentiation
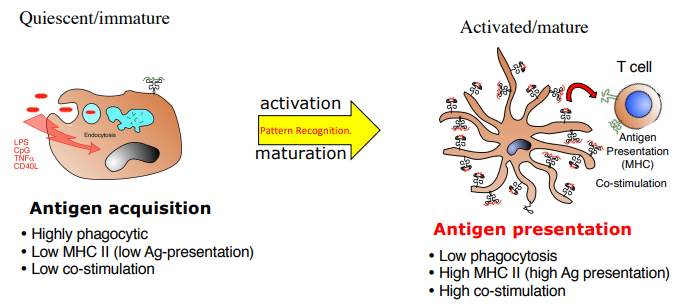
what are the 2 states of dendritic cells
immature: antigen acquisition
highly phagocytic
low MHCII
low co-stimulation
mature: antigen presentation (from activation via PRR)
low phagocytosis
high MHCII
high co-stimulation
what happens when a DC is activated by PAMPs
it migrates from the periphery to draining lymph nodes
adaptive immune responses are initiated by DC
what happens to a DC upon activation via pattern recognition
increase MHC expression & promotes antigen presentation
increase costimulation (increase CD80 & CD86)
promotes cytokine production