intro to aldehydes and ketones
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
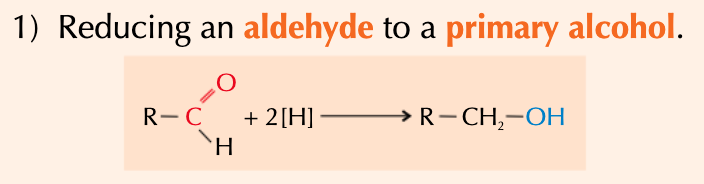
give the general eqn for the conversion of an aldehyde to a 1o alcohol - state the conditions and whether this is oxidation or reduction:
react w/ aq NaBH4 - acts as reducing agent
reduction
give the general eqn for the conversion of a ketone to a 2o alcohol - state the conditions and whether this is oxidation or reduction:
react w/ NaBH4 - acts as reducing agent
reduction

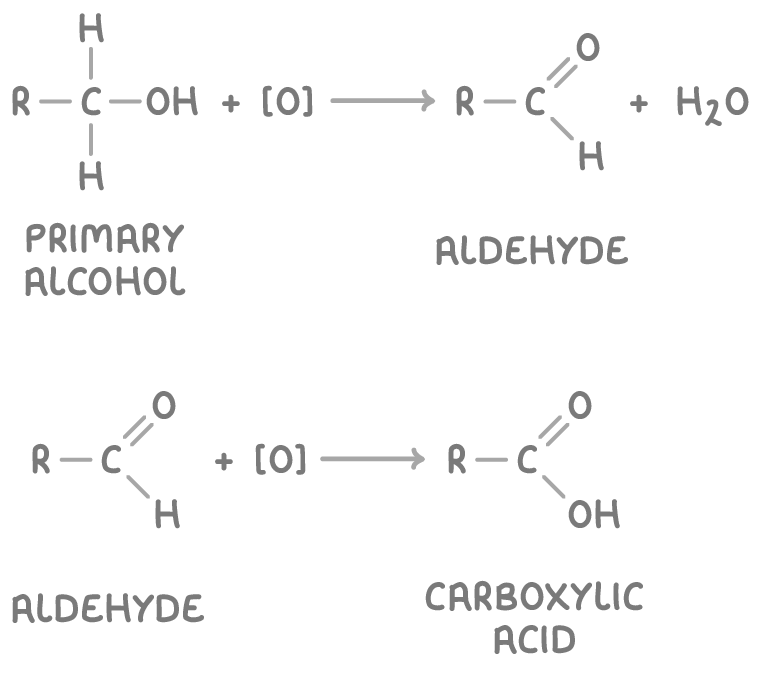
give the general eqns for the conversions to other organic compounds that may occur for a 1o alcohol - state the conditions and whether they are oxidation or reduction reactions:
both oxidation
1o alcohol oxidised to aldehyde
heat and distil w/ H2SO4 and Kr2Cr2O7 - latter is the oxidising agent
aldehyde can be oxidised further to a carboxylic acid using same reagents and heating under reflux OR heating w/ Tollen’s/Fehling’s
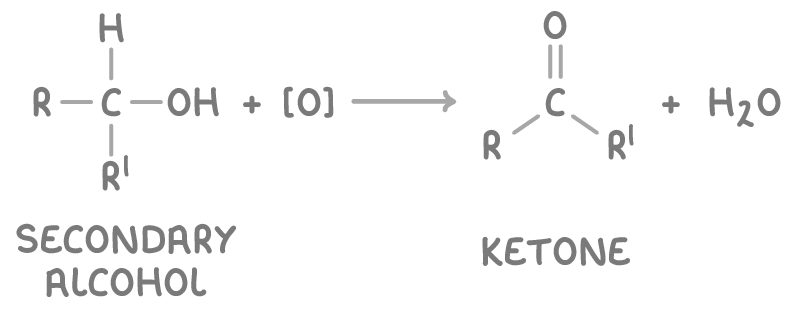
give the general eqn for the conversion of a 2o alcohol to a ketone - state the conditions and whether this is oxidation or reduction:
2o alcohol oxidised to ketone
heat under reflux w/ H2SO4 and K2Cr2O7 - latter is the oxidising agent
oxidation - cannot be oxidised further
give the eqn for and explain the colour change when 1o/2o alcohols are added to acidified potassium dichromate (VI):
orange dichromate (VI) ion is reduced to the green chromium (III) ion
Cr₂O₇²⁻ + 14H⁺ + 6e⁻ → 2Cr³⁺ + 7H₂O