Titration Curves
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Buffer
- They are usually made up equal concentrations of a weak acid and its weak conjugate base in an aqueous solution. - - The conjugate pair MUST be WEAK
- Now the system is resistant to any pH change caused by addition of a Strong acid or base
What is neutralization?
It is the mixing of equal mole portions of an acid with a base regardless of their concentrations and strengths
Neutralized acid or base: moles H3O+ = moles OH-
- Addition of base to acid (or acid to base) yields water and salt upon neutralization
THIS DOES NOT MEAN IT MAKES pH= 7
What is the neutralization point?
It is also referred to as the endpoint and equivalence point
What happens when the base is stronger than the acid?
Strong Base + Weak Acid
The neutralized solution is slightly basic, so pH is greater than 7
What happens when the base is weaker than the acid?
Strong Acid + Weak Base
The neutralized solution is slightly acidic, so pH is lower than 7
What happens when the base and acid are equally strong?
Strong Acid + Strong Base
The neutralized solution is neutral so pH = 7
What is the equivalence point for a weak acid?
For a weak acid titrated by a strong base, the equivalence point is the point at which it is completely converted into its conjugate base
The CB yields a pH greater than 7 so the pH greater than 7 at the equivalence point
What is the pH range of the buffer?
It is the pKa of the weak acid +/- 1
What happens to the pH of a buffer system if you add water to it?
The buffer pH remains the same since the water dilutes the concentration of the weak acid and weak conjugate base equally--> Means mole ratio of weak acid to weak CB does not change
So how do we know what buffer we need to choose?
1) You need to choose an acid with a pKa as close as possible to the desired pH (+/- 1 of the pH you want)
2) A mixture of HA and its CB A- should be formed with equal concentrations
Buffers at pH 2-5: Use carboxylic acids
Buffers at pH 8-11: Use amines
How are buffers made?
1) Combining a conjugate pair in roughly equal mole portions
2) Partial titration: partially titrating a weak acid with roughly half an equivalent of strong base or titrating a weak base with half equivalent of strong acid
What are the methods to mix buffers?
1) Weak Acid + salt of CB in equal concentrations
2) Weak Base + salt of CA in equal concentrations
3) Weak acid + half equivalent of strong base
4) Weak base + half equivalent of strong acid
When does buffering occur?
It only occurs with the titration of a weak reagent by a strong reagent
For ex: to be a buffer, the weak base (NH3) must be half-titrated by the strong acid (HCl)
When you titrate a weak acid, the buffering region is not entirely flat, but shows a slight pH change
In what states does a species exist if pH of environment is greater than the pKa of the species?
It is exists in its deprotonated state
In what states does a species exist if pH of environment is less than the pKa of the species?
It exists in its protonated state
What is respiratory acidosis?
When the carbon dioxide levels in the blood increase, causing the blood to be more acidic than normal
What is respiratory alkalosis?
When there is a loss of carbon dioxide in the blood
Metabolic alkalosis
When there is a loss of gastric fluids that are rich in HCl; this means there is a loss of acid and pH goes up
Metabolic acidosis
Loss of bicarbonate (HCO3 -) and blood pH goes down
When does the titration reach its equivalence point?
When the moles of acid equal the moles of base
What kinds of titration are there?
1) Strong reagent by a strong reagent
2) Weak reagent by a strong reagent
There can never be a weak reagent titrating a weak reagent
When a weak reagent is titrated by a strong reactant, what is the pH at the equivalence point?
The pH is greater or less than 7
With weak acid titrated by strong base pH > 7
With weak base titrated with strong acid pH <7
Graph of a weak acid being titrated with strong base
pH > 7 at the equivalence point because the neutralized product formed at equivalence is a weak base (CB of the weak acid)
The weaker the acid, the stronger the CB at the equivalence and therefore a higher pH
pH of conjugate base depends on concentration and Kb of acid at equivalence
Graph of a weak base being titrated with strong acid
pH < 7 at the equivalence point because the neutralized product formed at equivalence is a weak acid (CB of the weak base)
The weaker the base titrated, the stronger the CA at the equivalence and therefore a lower pH
pH of conjugate acid depends on concentration and Ka of acid at equivalence
When a strong acid is titrated by a strong base, what is the pH at the equivalence point?
The pH is = 7
Moles (HX) initial = Mols (OH-) added
The shape of the curve is referred to as sigmoidal
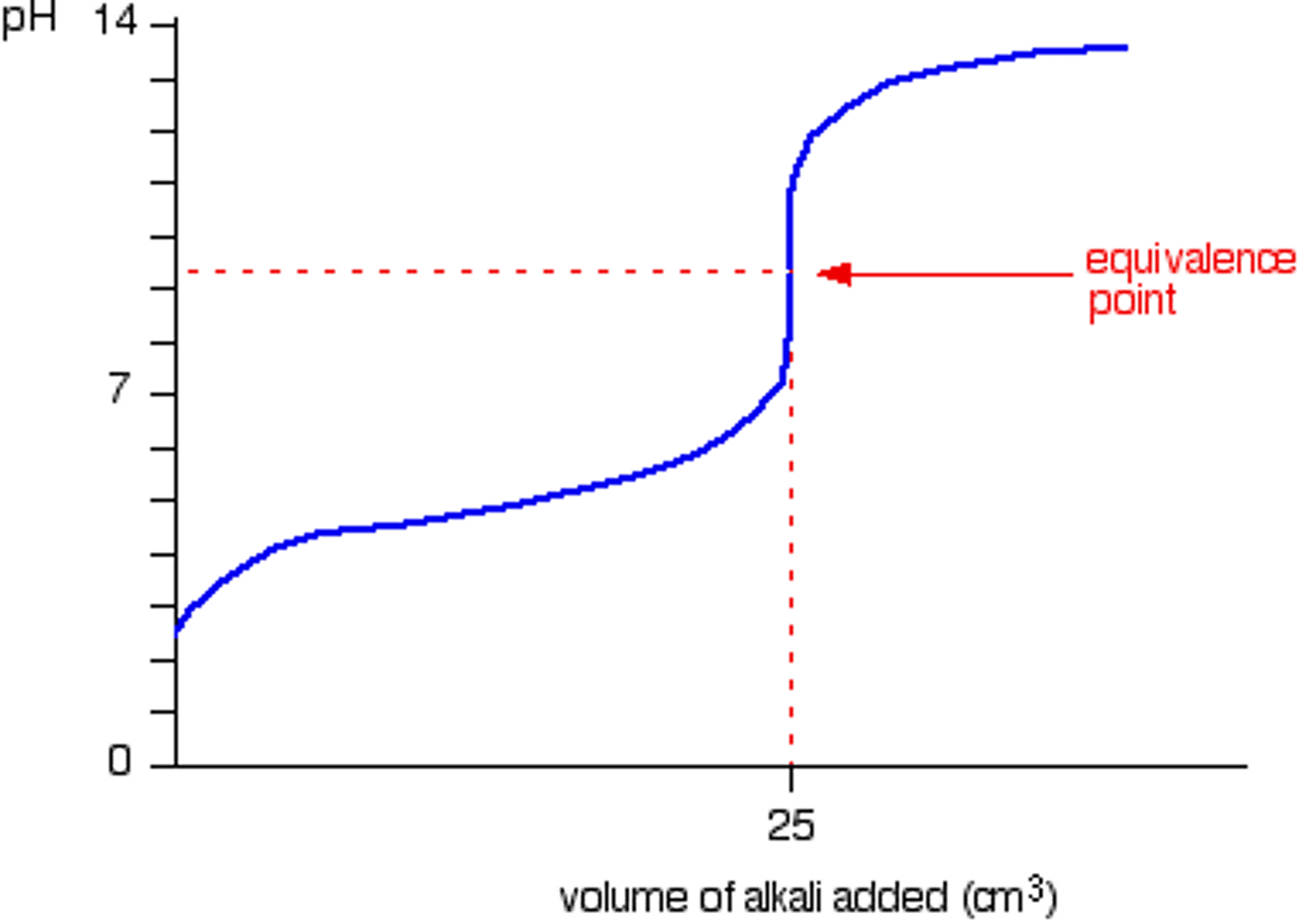
What happens to the concentration of hydronium when the pH increases from 1 to 2?
Since the pH goes up by one, and there is a log relationship, the concentration of hydronium decreases by a factor of 10.
Therefore, if it starts out as .10 M, it decreases to .01M meaning there was a change of .09 M
What is the pH at the half equivalence point for weak acids?
For weak acids, the pH = pKa at the half equivalence point
Because [HA] = [A-]
What is lip-o-weakness?
It is the initial climb in pH until buffering takes affect
What are the features of a titration curve where both reagents are strong?
1) an equivalence pH of 7
2) sigmoidal graph shape (The strong reagents fully dissociate, the highest concentration initially. Results in a slow change in pH until just before equivalence point)
3) the most rapid pH change near pH =7
4) pH is nearly constant in the beginning of titration
What are the features of a titration curve where one reactant is weak and titrant is strong?
1) Half equivalence pH = pKa
2) a pH at equivalence that is not equal to 7
3) a large change in pH as the first few drops of titrant are added
How does the concentration affect the titration curve?
The higher concentrations= Starts lower and ends higher
Higher base concentration leads to higher pH
What is the strength effect on titration curve shape?
As the acid becomes weaker, there is a larger initial lip, a higher midpoint where pH = pKa, and a greater equivalence point pH
As acid strength increases, the initial pH decreases, and size of lip-o-weakness decreases
What form is the indicator in when the pH of the solution is less than the pKa of the indicator?
The indicator is in the protonated state
What form is the indicator in when the pH of the solution is more than the pKa of the indicator?
The indicator is in the deprotonated state
Why do we use indicators?
1) First is to detect the endpoint of a titration
2) To approximate the pH of a solution from the color of the indicator in the solution
When does the indicator change color?
It changes color at a pH near the equivalence point of the titration
How to determine the pH at equivalence point of a weak acid being titrated by a strong base?
You take the average of the pKa of the weak acid and the pH of the strong base being added
The pKa of the indicator should be +/- this average
- As the titration carries out, the pH increases (addition of strong base) and the indicator goes from protonated to deprotonated form
How to determine the pH at equivalence point of a weak base being titrated by a strong acid?
You take the average of the pKa of the weak conjugate acid and the pH of the strong acid being added
The pKa of the indicator should be +/- this average
- As the titration carries out, the pH decreases (addition of strong acid) and the indicator goes from deprotonated to protonated form