Rates of Reaction and Chemical Equilibrium Concepts
1/127
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
128 Terms
Collision theory
Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy.
Activation energy
The minimum amount of energy that particles must have to react.
Factors that affect the rate of a reaction
Concentration, Temperature, Pressure, Catalyst, Surface area.
Equipment needed to measure the rate of reaction
Stop clock, Balance or measuring cylinder/gas syringe.
Loss of mass of the reactants
Use a balance to measure the loss of mass.
Volume of gas produced
Use a gas syringe or upturned measuring cylinder to measure the volume of gas produced.
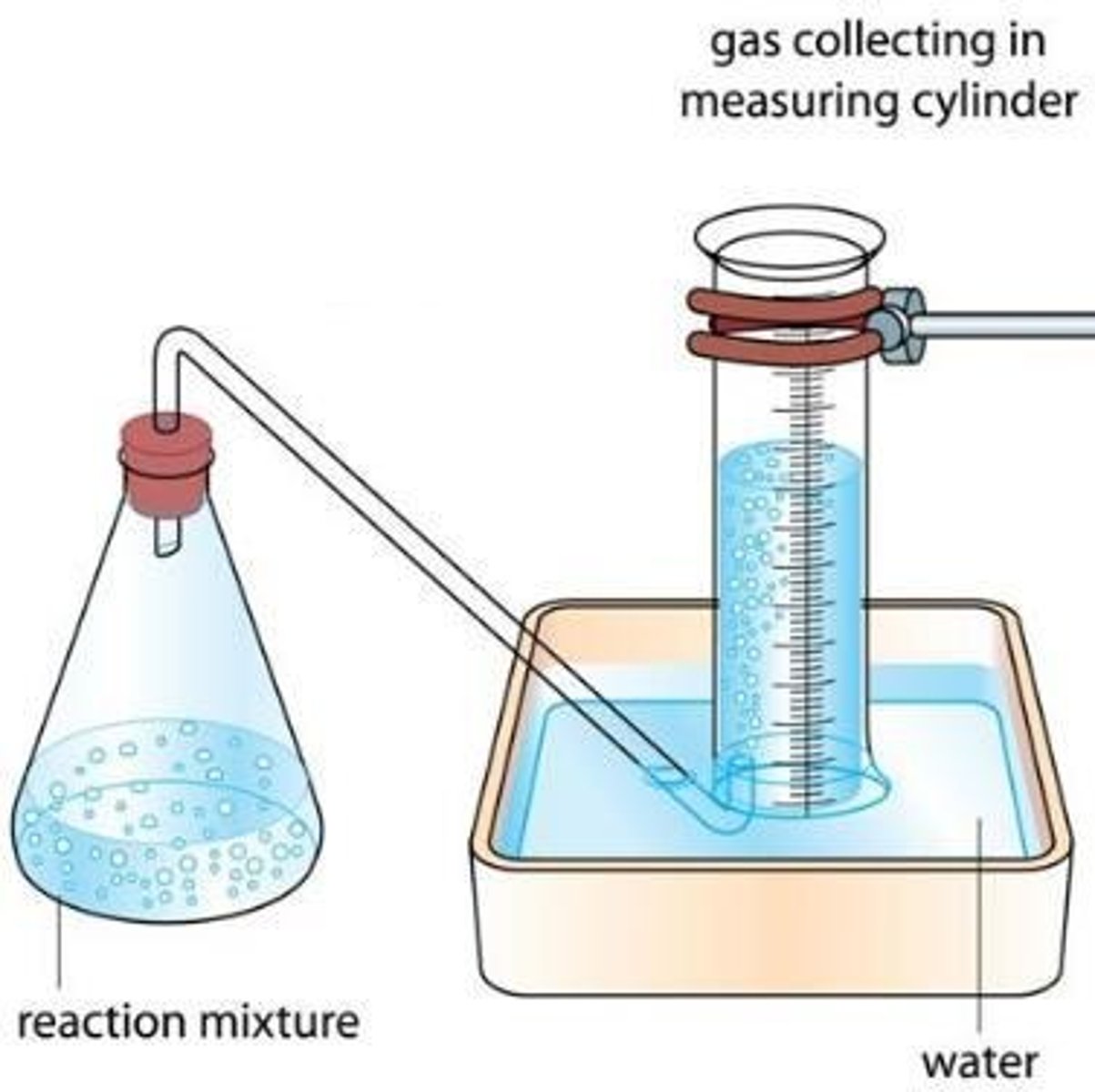
Time taken for the solution to become cloudy
Place conical flask on cross and watch it disappear.
Rate calculation at a specific time
Rate = change in mass / change in time.
Average rate calculation
Rate = change in y / change in x.
Gradient calculation
Gradient = change in y / change in x.
Rate example calculation
Rate = (3.1 - 1.4) / (120 - 30) = 0.0189 /s.
Steeper curve on rate graphs
Indicates a faster rate of reaction.
Horizontal line on graph
Indicates that the reaction is finished and reactants are used up.
Limiting factor
Reaction stops due to a limiting factor.
Closed system
When reactants or products cannot enter or leave the system.
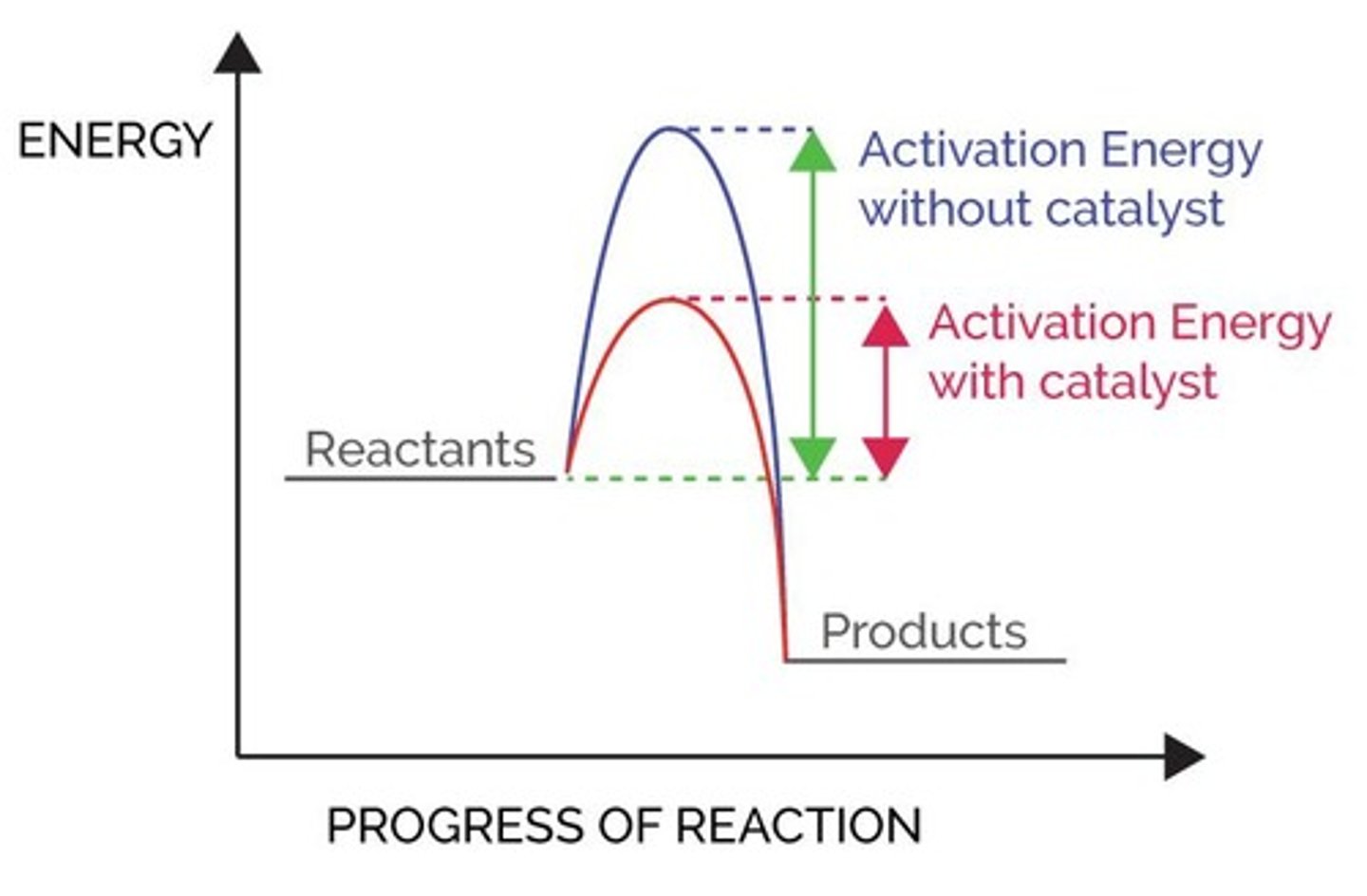
Catalysts
Increase the rate of reaction by providing a different pathway for the reaction that has a lower activation energy.
Equilibrium definition
The rate of the forward and reverse reaction is the same, and the concentrations of reactants and products are constant.
Le Chatelier's principle
Use to explain the effect of changing the conditions on the position of equilibrium.
Biological catalyst
Enzyme.
Effect of temperature on equilibrium
Increase in temperature - reaction moves in the endothermic direction; Decrease in temperature - reaction moves in the exothermic direction.
Effect of pressure on equilibrium
Increase in pressure - reaction moves to the side of the fewer moles; Decrease in pressure - reaction moves to the side of the most moles.
Effect of concentration on equilibrium
Increase in concentration of a chemical - reaction moves to the opposite side to use up excess chemical; Decrease in concentration - moves to this side to create more of this chemical.
Reversible reaction example
Anhydrous copper sulfate + water → Hydrated copper sulfate.
Effect of a catalyst on equilibrium
No effect on the position of equilibrium; allows the reaction to reach equilibrium faster.
Evolution of the Atmosphere
Methane reacted with oxygen to form carbon dioxide and water; Volcanoes released water vapour, carbon dioxide, methane, ammonia.
Today's atmosphere composition
78 % Nitrogen (N2), 21 % oxygen (O2), 1 % other gases.
Reasons why O2 levels increased
Oxygen levels increased, allowing animals to evolve; Absorbed by oceans.
Reasons why CO2 levels decreased
Locked up as sedimentary rocks and fossil fuels; Used in photosynthesis to produce oxygen.
Formation of coal
Carbon dioxide was used during photosynthesis by trees; Trees die and are compressed over millions of years.
Greenhouse Gases
Water vapour (H2O), Carbon dioxide (CO2), Methane (CH4) that trap heat in the atmosphere.
Effects of Global Climate Change
Includes sea level rise, more frequent and severe storms, changes to wildlife distribution, and increased temperatures.
Human Activities Which Increase Greenhouse Gases
Combustion of fossil fuels, deforestation, and increased animal farming contribute to higher greenhouse gas emissions.
Carbon Footprint
The total amount of carbon dioxide and other greenhouse gases emitted over the full life cycle of a product, service or event.
How to Reduce the Carbon Footprint
Increased use of alternative energy supplies, energy efficient appliances, carbon capture and storage (CCS), and lifestyle changes.
Problems on Reducing the Carbon Footprint
Economic considerations such as the affordability of building more wind turbines.
Sulfur dioxide (SO2)
Formed from sulfur in fossil fuels reacting with oxygen; causes respiratory problems and acid rain.
Carbon monoxide (CO)
Produced from incomplete combustion of hydrocarbons; a toxic gas that can cause death.
Carbon particulates (unburned hydrocarbons)
Solid particles resulting from incomplete combustion that can harm health and the environment.
Oxides of nitrogen (NOx)
Formed from nitrogen and oxygen reacting at high temperatures; causes respiratory problems and acid rain.
Mole
Mole = mass (g) / relative formula mass.
Avogadro's Number
6.02x10^23, the number of particles in one mole of a substance.
Volume
Volume in dm3 is calculated by dividing cm3 by 1000.
Concentration
Concentration (g/dm3) = mass (g) / volume (dm3).
Limiting Reactants
The reactant that is completely used up in a reaction, limiting the amount of products formed.
Theoretical Yield Calculation
The mass of product expected from a reaction based on the limiting reactant.
Example of Limiting Reactants
In the reaction 2H2O2 → 2H2O + O2, the limiting reactant is determined by comparing moles.
Mass of Oxygen Produced
128 grams of hydrogen peroxide breaks down into water and oxygen.
Moles Calculation
Moles = mass / relative atomic mass; for example, 3 g of Mg reacts with 7 g of O2.
Reaction Ratio
The ratio of reactants and products in a chemical reaction, such as 2H2O2 : 1O2.
Final Reaction Completion
Once the limiting reactant has reacted, the reaction is complete.
Photosynthesis
The process by which trees use carbon dioxide to produce oxygen and reduce global warming.
Decomposition of Rubbish
Leads to increased methane emissions from landfill sites.
Animal Farming
Contributes to methane emissions through digestion and waste decomposition.
Balanced Equation
The equation representing the reactants and products in a chemical reaction with equal numbers of atoms for each element.
Moles of Reactants and Products
The quantity of a substance measured in moles, used to balance chemical equations.
Alkane
A hydrocarbon made of C-C single bonds.
Alkane General Formula
Cn H2n + 2, where n is the number of carbon atoms.
Homologous Series
A family of hydrocarbons with similar chemical properties who share the same general formula.
Fractional Distillation
A process to separate mixtures based on different boiling points.
Boiling Point
The temperature at which a liquid turns into a gas.
Flammability
How easily a substance ignites (catches on fire).
Viscosity
The runniness of a liquid; higher viscosity means longer flow time.
Volatility
How easily a liquid changes into a gas.
Incomplete Combustion
A reaction with insufficient oxygen, producing carbon monoxide and water.
Complete Combustion
A reaction with sufficient oxygen, producing carbon dioxide and water.
Cracking
The process of breaking down hydrocarbons into smaller, more useful molecules.
Thermal Decomposition
Breaking down a compound using heat.
Catalytic Cracking
Cracking that requires a catalyst and high temperature.
Steam Cracking
Cracking that uses high temperature and steam.
Alkene
A hydrocarbon made of C=C double bonds.
Chemical Test for Alkene
Add bromine water; alkene turns from orange to colourless.
Test for Carbon Dioxide
Bubble through limewater; result turns cloudy.
Fossil Fuels
Non-renewable fuels such as coal, crude oil, and natural gas.
Combustion
Burning in oxygen.
Fraction
Molecules with a similar number of carbon atoms.
Reaction Conditions
The specific conditions under which a chemical reaction occurs.
Carbon Monoxide
A toxic gas produced during incomplete combustion.
Gas
Chemical test
Hydrogen (H2)
Lit splint; Pop sound
Oxygen (O2)
Glowing splint; Splint relights in oxygen.
Formulation
A mixture that has been designed as a useful product e.g. shampoo.
Carbon Dioxide (CO2)
Turns milky/cloudy when bubbled through limewater.
Chlorine (Cl2)
Damp litmus paper; Paper is bleached (white).
Pure water
Boil it; Boils at exactly 100 oC.
Melting point
The temperature at which a solid turns into a liquid. Ice has a melting point of 0 oC.
Chromatography
Can be used to separate mixtures and identify substances. Relies on the difference in solubility of the mixture.
Mobile phase
The solvent e.g. water running up the chromatogram.
Stationary phase
The paper used in chromatography.
Rf value
Tells you how far the substance has moved, relative to the solvent. Rf = distance moved by substance / distance moved by solvent. Rf value will always be less than 1.
Potable water
Safe to drink. Contains low levels of dissolved salts and microbes. Not pure.
Finite resource
A source from the Earth that is running out e.g. coal, crude oil.
Renewable source
A source that isn't running out e.g. wood.
Distillation
Method to obtain potable water from salty water by heating, evaporating, cooling, and condensing.
Sterilising agents
Chlorine, ozone, or ultra-violet light used to kill microbes.
Life Cycle Assessments (LCAs)
To assess the environmental impact of the stages in the life of a product.
High grade copper ore
Rock that contains enough copper that makes it economically viable to extract.
Low grade copper ore
Extract using phytomining or bioleaching.
Bioleaching
Uses bacteria to produce leachate solutions that contain metal compounds.
Saving Resources
Limits the use of raw materials, energy consumption, waste and environmental impacts.
Reuse
Use the item for another purpose e.g. a glass bottle is refilled.