Exp 1 - Crystallization + Melting Point
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
crystallization is a technique used for
purification of solids with impurities
Crystallization is based on the ___ difference between ___
the solubility difference between the solid and the impurities
what factors affect crystallization?
concentration, temperature, polarity
the ideal solvent for the crystallization technique is one that…
dissolves the sample at a high temperature but not at a low one
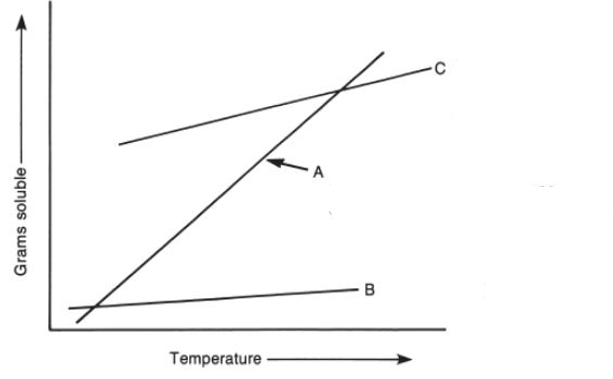
which is the best solvent?
A, because it is very soluble at higher temps and not soluble at lower temps
how does crystallization work?
impure solid is dissolved in a small amount of hot solvent. now, the concentration of the solid is high, while the concentration of impurities is low.
when the solution cools, the solid precipitates as crystals, while impurities remain in solution.
the crystals are filtered and dried, and are now isolated
critical factors of the solvent in crystallization are…
polarity and boiling point
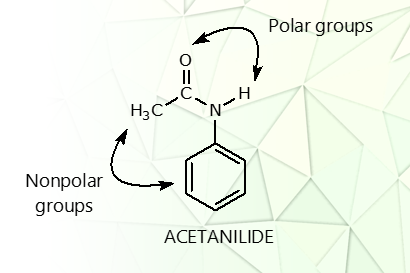
what are the polar groups on acetanilide?
the carbonyl and the amide groups
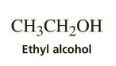
what solvent are we using?
ethanol
in this experiment, everything goes in the ___ waste
organic
the solution from which the crystals are obtained is referred to as the ___
mother liquor (or the supernatant)
how do you calculate percent yield?
(actual yield)/(theoretical) x 100%
compounds possessing O or N are ___
polar
polar/nonpolar? why
polar, because of the electronegative oxygen
the most polar compounds are capable of forming ___
hydrogen bonds
ethanol is (more/less) polar than water
less
does ethanol form hydrogen bonds?
yes
after the crystals are removed, the mother liquor contains the ___
impurities
why is the ice bath used?
to further lower the temperature and increase the crystal yield.
cooling the mixture increases the yield by decreasing ___
the solubility of the substance
how do you wash the crystals?
by adding a small amount of cold solvent. this removes any mother liquor adhered to their surface. the crystals then dry in the funnel, then in a watchglass.