Proteins
0.0(0)
Card Sorting
1/217
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
218 Terms
1
New cards
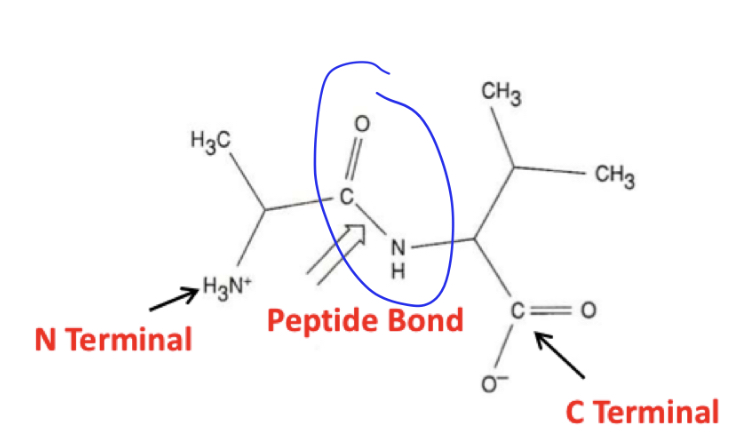
What are polymers of amino acids linked together by?
peptide bonds
2
New cards
Molecular weight < 10k
peptides
3
New cards
Molecular weight > 10k
proteins
4
New cards
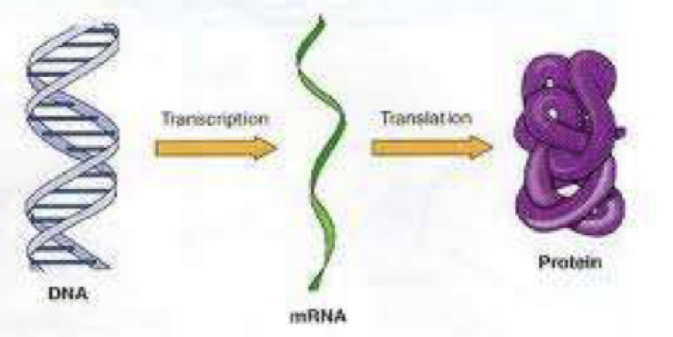
How are proteins related to genetic expression
they are the end products
5
New cards
What are proteins composed of?
at least **one carboxylic acid**, and **one amino group** attached to an adjacent ==alpha-carbon==
6
New cards
What are bigger, peptides or proteins?
Proteins
7
New cards
Why are proteins good buffers?
Have a great balance of charge due to their structure
8
New cards
What is transcription?
Making an mRNA copy of a DNA strand
9
New cards
What is translation?
Using an mRNA to make a protein
10
New cards
General functions of proteins
* mechanical support
* regulate growth and differentiation
* transportation & storage
* catalysts
* motion
* nerve propagation and immune protection
* intra/extra cellular buffers
* regulate growth and differentiation
* transportation & storage
* catalysts
* motion
* nerve propagation and immune protection
* intra/extra cellular buffers
11
New cards
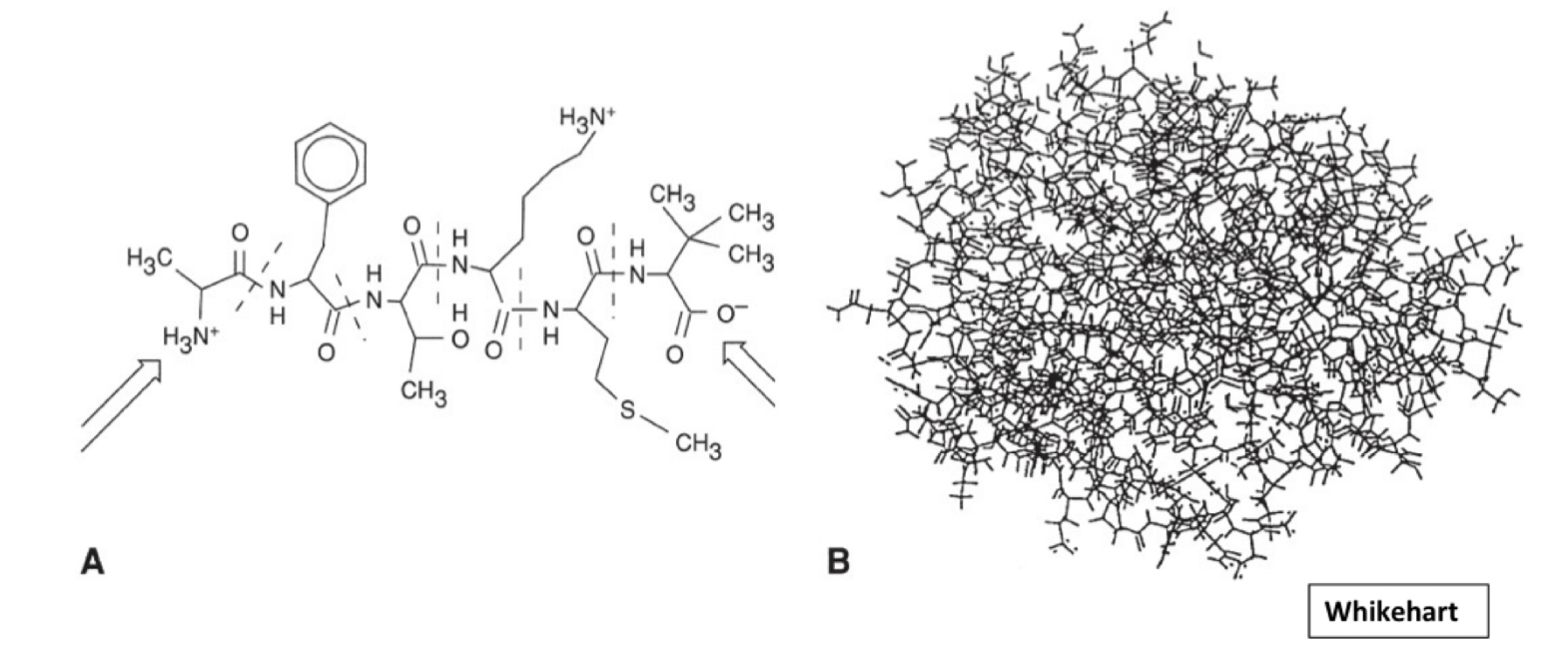
What structure is A? B?
A: peptide
B: protein (more complex, larger)
B: protein (more complex, larger)
12
New cards
Functions of proteins in the eye
* support structure and clarity of cornea
* variable light refraction of the lens
* initiative transduction of light into electrical signal
* generate IOP
* lyse bacteria in precorneal tear film
* variable light refraction of the lens
* initiative transduction of light into electrical signal
* generate IOP
* lyse bacteria in precorneal tear film
13
New cards
Solubility classifications of proteins
* water-soluble
* lipid-soluble
* insoluble
* lipid-soluble
* insoluble
14
New cards
Unsubstituted amino acid
glycine
15
New cards
Dicarboxylic amino acid
aspartic acid
16
New cards
Diamino amino acid
Lysine
17
New cards
Amido amino acid
Asparagine
18
New cards
Hydroxy amino acid
serine
19
New cards
Aromatic amino acid
phenylalanine
20
New cards
Sulfur amino acids
cysteine
21
New cards
Cyclic amino acid
Proline
22
New cards
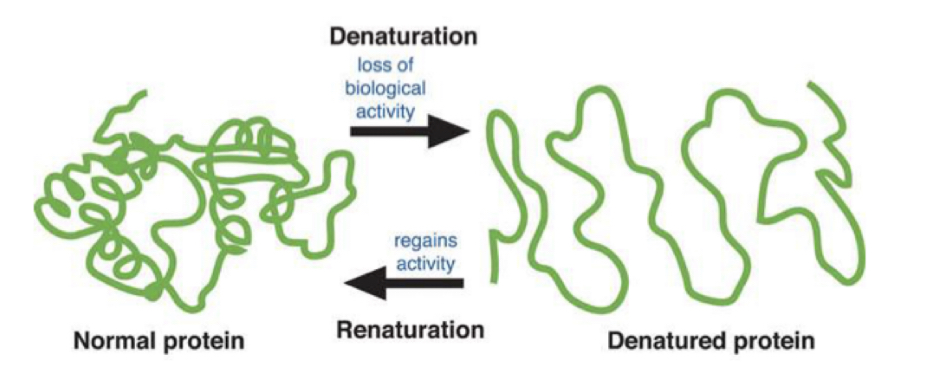
What happens when proteins are denatured?
can lose their function temporarily or permanently
23
New cards
What happens when proteins are denatured?
change in conformation of the native form of the protein
24
New cards
What are causes of protein denaturation?
* change of temperature
* ionic composition
* environmental factors
* ionic composition
* environmental factors
25
New cards
What type or proteins are more prone to denaturation?
Catalytic proteins
26
New cards
What are the 4 basic structures of proteins?
1. protein
2. secondary
3. tertiary
4. quaternary
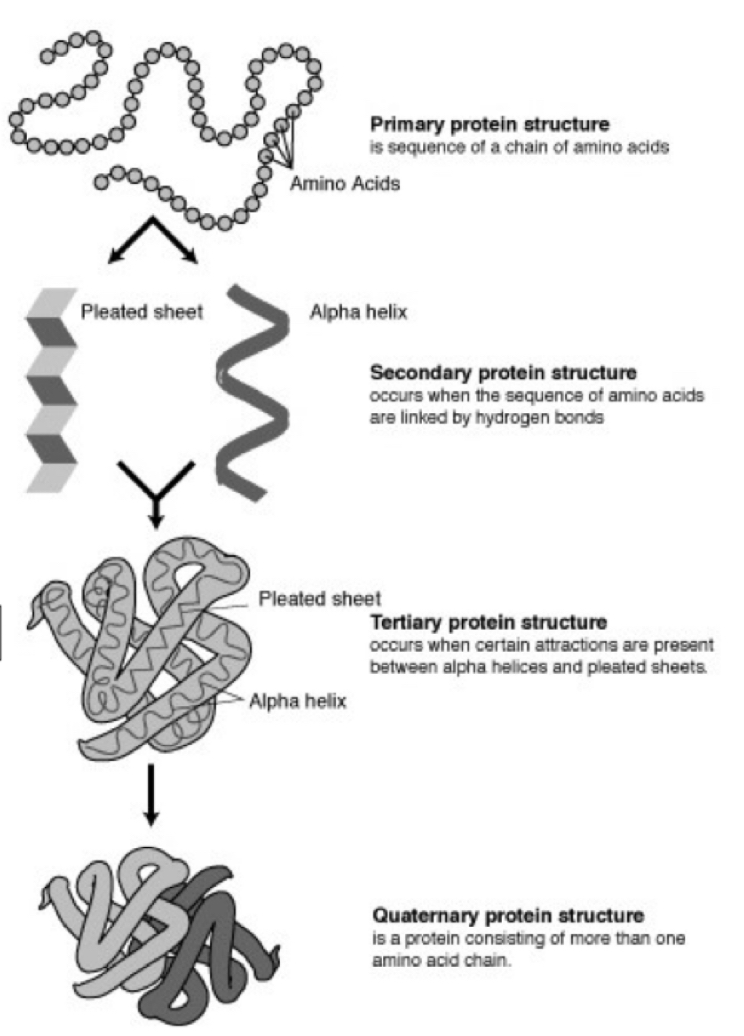
27
New cards
What is a primary protein structure?
chain of amino acids
28
New cards
What is a secondary protein structure?
When sequence of amino acids are linked by **hydrogen bonds**, creating pleated sheets or alpha helix
29
New cards
What is the structure of a tertiary protein?
when there are attractions present between pleated sheets/alpha helix (secondary protein)
30
New cards
What is the structure of a quarternary protein?
consisting of more than one amino acid chain
31
New cards
How many configurations/shapes does each amino acid sequence assume?
4
32
New cards
Why would a protein change in configuration?
* bulkiness
* change in density
* hydrophobic regions of amino acids
* change in density
* hydrophobic regions of amino acids
33
New cards
What determines the shape of a proteins?
* hydrogen bonding
* disulfide bonding within and between amino acid chains
* disulfide bonding within and between amino acid chains
34
New cards
antiparallel
sequence parallel to another sequence, but with opposite alignment
35
New cards
What are the types of structures of secondary protein?
1. random coils: irregular forms
2. Beta-turns: 180 turns that connect beta-pleated sheets
3. Beta-pleated sheets: parallel or antiparallel sequence of primary structures
36
New cards
What are the structures of secondary proteins held together by?
numerous hydrogen bonds
37
New cards
What is a domain?
specialized region in a protein having specific function
38
New cards
What is a motif?
simplified version of a domain
39
New cards
What structure is most common in soluble and transmembrane proteins?
alpha-helix structure
40
New cards
what are alpha-helices and beta-pleated sheets stabilized by?
hydrogen bonds
41
New cards
What type of structure is Rhodopsin mostly?
alpha-helical
42
New cards
What do quarternary structures of proteins consists of?
two or more polypeptide chains
43
New cards
Describe an immunoglobulin structure
* 3D
* 2 light and 2 heavy chains
* **disulfide bonds/sulfur bridges**
* 2 light and 2 heavy chains
* **disulfide bonds/sulfur bridges**
44
New cards
Function of lysozyme
lyses the peptidoglycan cell wall of **gram + bacteria in tear film**
45
New cards
Rhodopsin
protein found in the outer segments that mediates visual transduction
46
New cards
Post-translational modification
alteration of the peptide chain of proteins after synthesis
47
New cards
Significance of protein function to the eye
* source for generation of osmotic pressure across cell boundaries
* corneal deturgescence
* generation of IOP
* corneal deturgescence
* generation of IOP
48
New cards
What is visual transduction?
conversion of light energy to electrical energy
49
New cards
What are crystallins
group of structural proteins in the crystalline lens
50
New cards
Where are crystallins found in the lens?
epithelial and fiber cells of the ocular lens
51
New cards
What solubility type of protein are crystallins?
water soluble
52
New cards
Why are crystallins important for our vision?
when they maintain elongated shape, **they affect the refraction of light**
53
New cards
How many types of crystallins are found in the lens?
1. alpha
2. beta
3. gamma
54
New cards
subtypes of alpha crystallins
1. alpha-A
2. A-beta
55
New cards
subtypes of beta crystallins
1. beta-H
2. beta-L
56
New cards
subtypes of gamma crystallins
gamma A-F
57
New cards
Role of alpha crystallins
* molecular chaperones
* maintain the normal molecular conformation
* preventing crystallin aggregation (senile cataracts)
* inhibits light scattering
* maintain the normal molecular conformation
* preventing crystallin aggregation (senile cataracts)
* inhibits light scattering
58
New cards
What are senile cataracts?
newly forming cataracts, when the crystallins are beginning to lump together
59
New cards
What type of crystallin is the best chaperone?
alpha crystallin
\
* helps fold and stabilize beta and gamma crystallins
\
* helps fold and stabilize beta and gamma crystallins
60
New cards
How is crystallin concentration different in cells?
concentration is **twice** that of most intracellular proteins (33% vs 15%)
61
New cards
When alpha and beta crystallins are made, are they stable?
yes, because they are **acetylated**
62
New cards
Why is acetylation important?
prevents cellular depredation of proteins
63
New cards
How can alpha and beta crystallins be altered?
* phosphorylation
* incorporation of sugars
* deamidation/degredation of the polypeptide chain
* incorporation of sugars
* deamidation/degredation of the polypeptide chain
64
New cards
What is the secondary structure of all human crystallins?
beta-pleated sheets
65
New cards
What structure do some alpha crystallins have?
helical structure
66
New cards
describe the structure of a gamma crystallin
* two sets of motifs that are aligned in a V shaped
* **two domains for each gamma crystallin**
* hydrophilic on outside, hydrophobic inside
* **two domains for each gamma crystallin**
* hydrophilic on outside, hydrophobic inside
67
New cards
Why are **methionine (102)** and **cysteine (109)** important to point out in gamma crystallins?
they are potential locations of oxidation of protein, which can cause opacities in lens
68
New cards
Why does oxidation bad in the lens, and what does it cause?
promotes crystallin aggregation, can cause cataracts
69
New cards
Describe the structure of alpha crystallins
* two bound rings of crystallin subunits laid on top of each other
* large units - hydrophilic
* small units - hydrophobic
* large units - hydrophilic
* small units - hydrophobic
70
New cards
Where do the chaperon activities of alpha crystallins occur?
at the extensions that cover the central cavity
71
New cards
How many alpha crystallin subunits are there
40 different subunits
72
New cards
What crystallin(s) is/are found in the lens epithelium
ONLY alpha crystallins
73
New cards
What crystallin(s) is/are found in lens fiber cells?
all three crystallins
74
New cards
Can lens proteins be renewed?
No, they are not metabolically renewed in the lens fiber cells
75
New cards
What are the steps in the transformation process of crystallins when aging occurs
1. phosphorylation
2. disulfide bond formation
3. deamidation
4. peptide bone disruption
76
New cards
What happens during phosphorylation in lens aging?
amount of phosphorylation chains of alpha crystallins increases
\
this causes negative charge of the protein to increase
\
this causes negative charge of the protein to increase
77
New cards
Describe disulfide bond formation what type of process is disulfide bond formation?
oxidative process
78
New cards
Why are cysteine groups important when talking about disulfide bond formation?
cysteine groups are a potential source causing the bonds to form
79
New cards
Why is gamma crystallins the most unstable?
they have the highest concentration of cysteine
80
New cards
What is deamidation?
loss of amide group
81
New cards
What is an example of deamidation in the lens?
aspargine and glutamine are converted to their corresponding dicarboxylic acids
82
New cards
What is peptide bond disruption?
when peptide bonds break in crystallins
83
New cards
Where does the peptide bond break in beta crystallins
near the N terminal region
84
New cards
Where does the peptide bond break in gamma crystallins?
near the C terminal region
85
New cards
What causes peptide bond disruption?
endopeptidase activity
86
New cards
What change in concentrations causes cataracts?
large increase of water soluble protein fraction after age 50
87
New cards
What is the insoluble fraction in an individual 80+ years old
50% or more
88
New cards
In normal aging process, describe cataract formation
* insoluble fraction increases
* disulfide bonding (cysteine), crosslinking
* disulfide bonding (cysteine), crosslinking
89
New cards
what are high molecular weight aggregates?
aggregated of crystallins
90
New cards
How do we term the HM aggregates of crystallins?
HM1, HM2, HM3, HM4
91
New cards
Which HMWA of crystallins are not considered threats for cataracts?
HM1 and HM2 because they are **soluble complexes**
92
New cards
Which HMWA of crystallins are considered a threat for cataracts?
HM3 and HM4 because they are **insoluble complexes**
93
New cards
Describe HM3
* **held together by disulfide bonds**
* mol wt: 2-33 mil. daltons
* associated with cortical cataracts
* noncrystallin protein
* mol wt: 2-33 mil. daltons
* associated with cortical cataracts
* noncrystallin protein
94
New cards
Describe HM4
* **do not involve disulfide bonds**
* associated with dark color of nuclear cataracts
* associated with dark color of nuclear cataracts
95
New cards
Difference of nuclear cataract and cellular debris formation
formation of disulfide, covalent bonds leads to cellular debris formation
\
\*\*\****nuclear cataract related to HM4 which DOES NOT have disulfide bonds***
\
\*\*\****nuclear cataract related to HM4 which DOES NOT have disulfide bonds***
96
New cards
Cortical cataract mechanism
1. normally folded crystallins protect oxidizable amino acids
2. hydrogen peroxide in aqueous can oxidize methionine and denature protein
3. exposed cysteines oxidize and form disulfide bonds with other crystallins and membrane proteins
97
New cards
Why is glutathione important? Is having less glutathione good or bad?
* protects crystallins from crosslinking by binding exposed groups like cysteine
* less = bad
* less = bad
98
New cards
What is the role of tryptophan?
* forms component of crystallins
* thought to be associated with nuclear cataracts
* **may contribute to the increase in yellow coloration of the lens with age**
* thought to be associated with nuclear cataracts
* **may contribute to the increase in yellow coloration of the lens with age**
99
New cards
What is the influence of UV light on the lens?
* oxidizes tryptophan to N-formylkynurenine
* contributes yellow/brown coloration of nuclear cataracts
* contributes yellow/brown coloration of nuclear cataracts
100
New cards
What type of cataracts are associated with “nondisulfide crosslinking characteristic of HM4 aggregates”?
nuclear cataracts