Chemistry - History of the Atom
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Dalton’s contributions to the atomn
indivisible atoms (cannot be broke down)
no positive or negative (no charges/subatomic particles)
Dalton’s picture of the atom
Thomson’s contributions to the atom
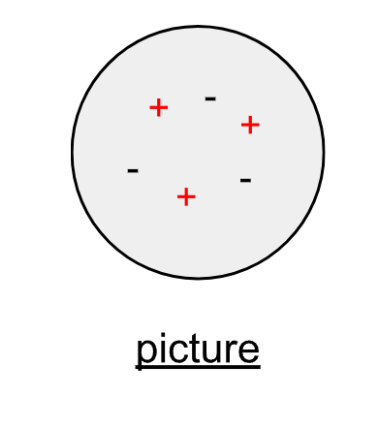
atom has positive and negative charges inside
net charge of zero
equal number of subatomic particles
Thomson’s picture of the atom
Rutherford’s contributions
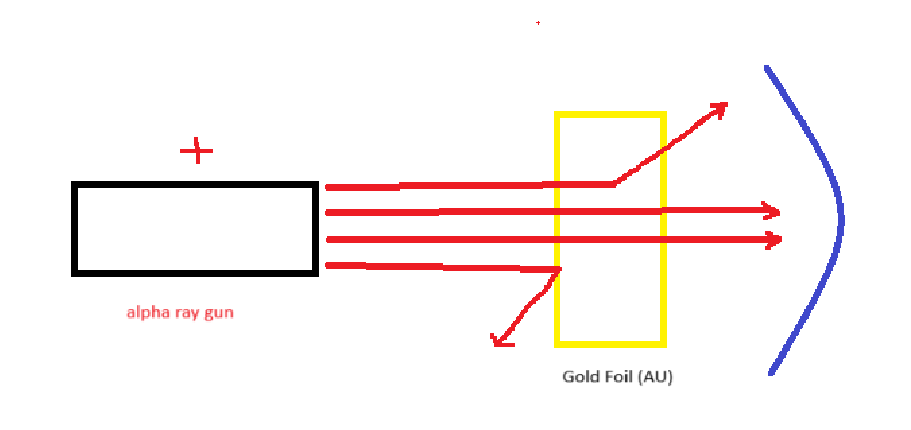
gold foil experiment
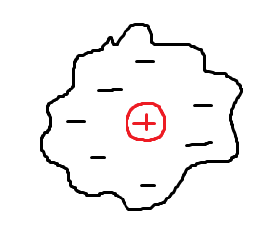
pos alpha beans would go through the gold foil since atoms are neutral
concluded that positive nucleus and cloud of electrons
Rutherford’s picture of the atom
Two flaws of Rutherford’s atom?
not organized
positive and negative attract, so the cloud theory does not make sense
Why did some of the rays deflect in the gold-foil experiment?
since the nucleus is positive, the rays would deflect off the positive nucleus
Gold Foil Experiment
Bohr’s contributions to the atom
electrons orbit the nucleus in energy levels
Why did Bohr question Rutherford’s atom?
Questioned the attraction between the positive nucleus and the “cloud” of negative electrons.
How many electrons in 1st shell?
2
how many electrons for the 2nd shell
8
how many electrons for the 3rd shell
8
protons =
atomic number
neutrons =
atomic mass - atomic number
Bohr’s picture of the atom
What is an ion
an atom with a charge due to a loss or gain of electrons
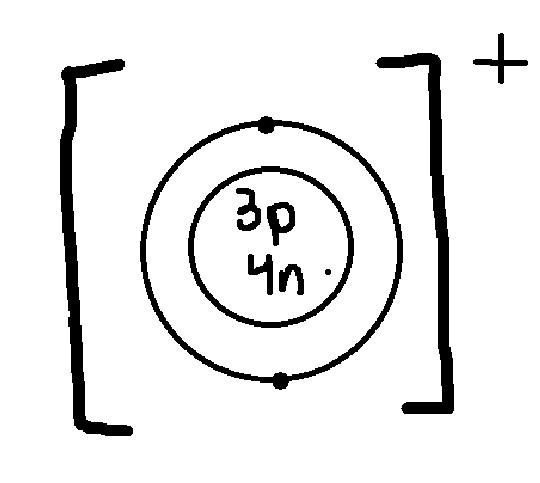
What does the + for this lithium ion mean?
it lost an electron, so it is positively charged
How do you do lewis dot diagrams?
use the # of valence electrons (group #)