Isotopes, Protons, Nuetrons, Electrons
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
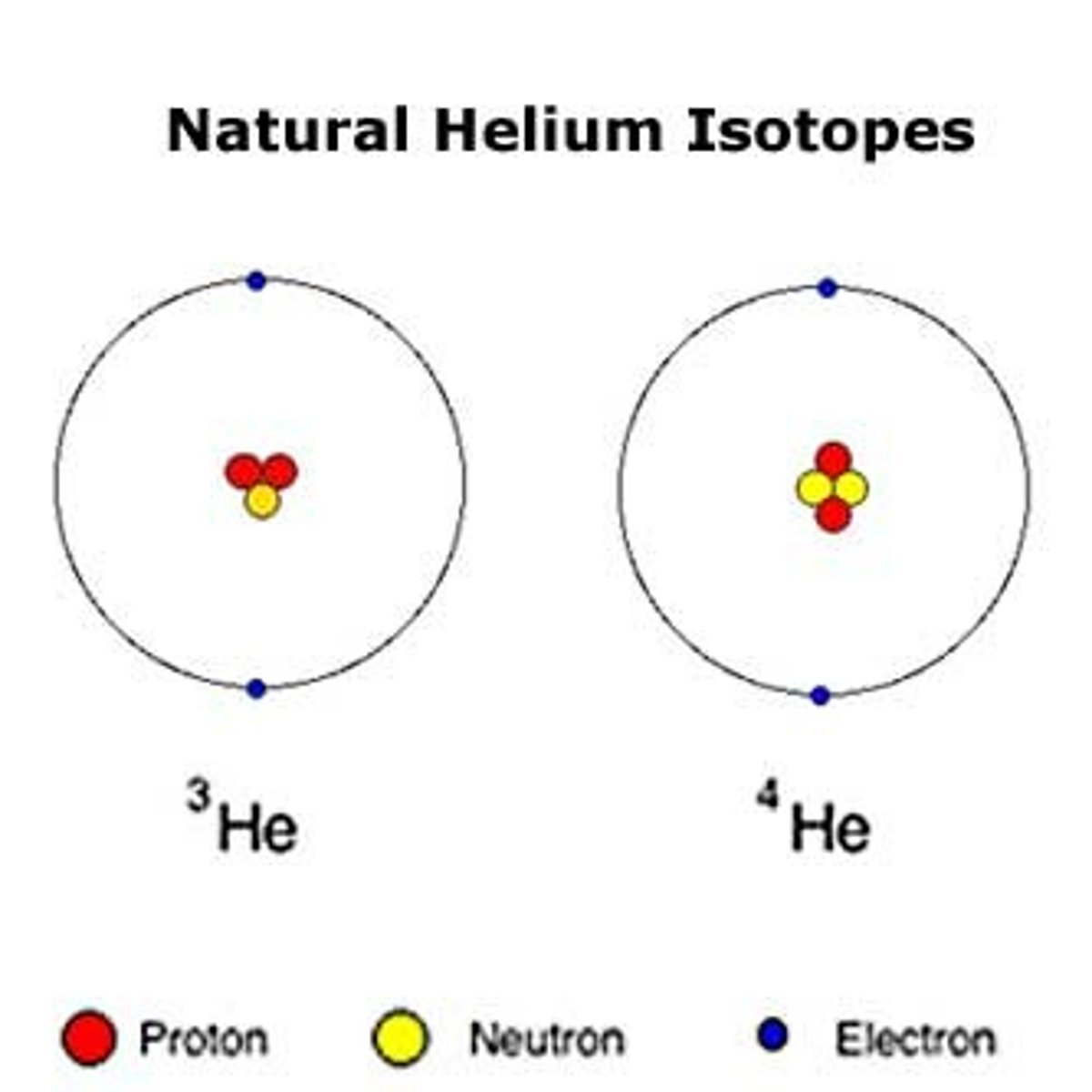
Isotope
Atoms with the same number of protons, but different numbers of neutrons.
7
Mass number

3
atomic number

protons plus neutrons
mass number of an isotope
charge = protons - electrons
how to find atom charge
mass number - protons
number of neutrons in an isotopes
atomic number of element
protons in an isotope
neutrons
no charge, found in nucleus
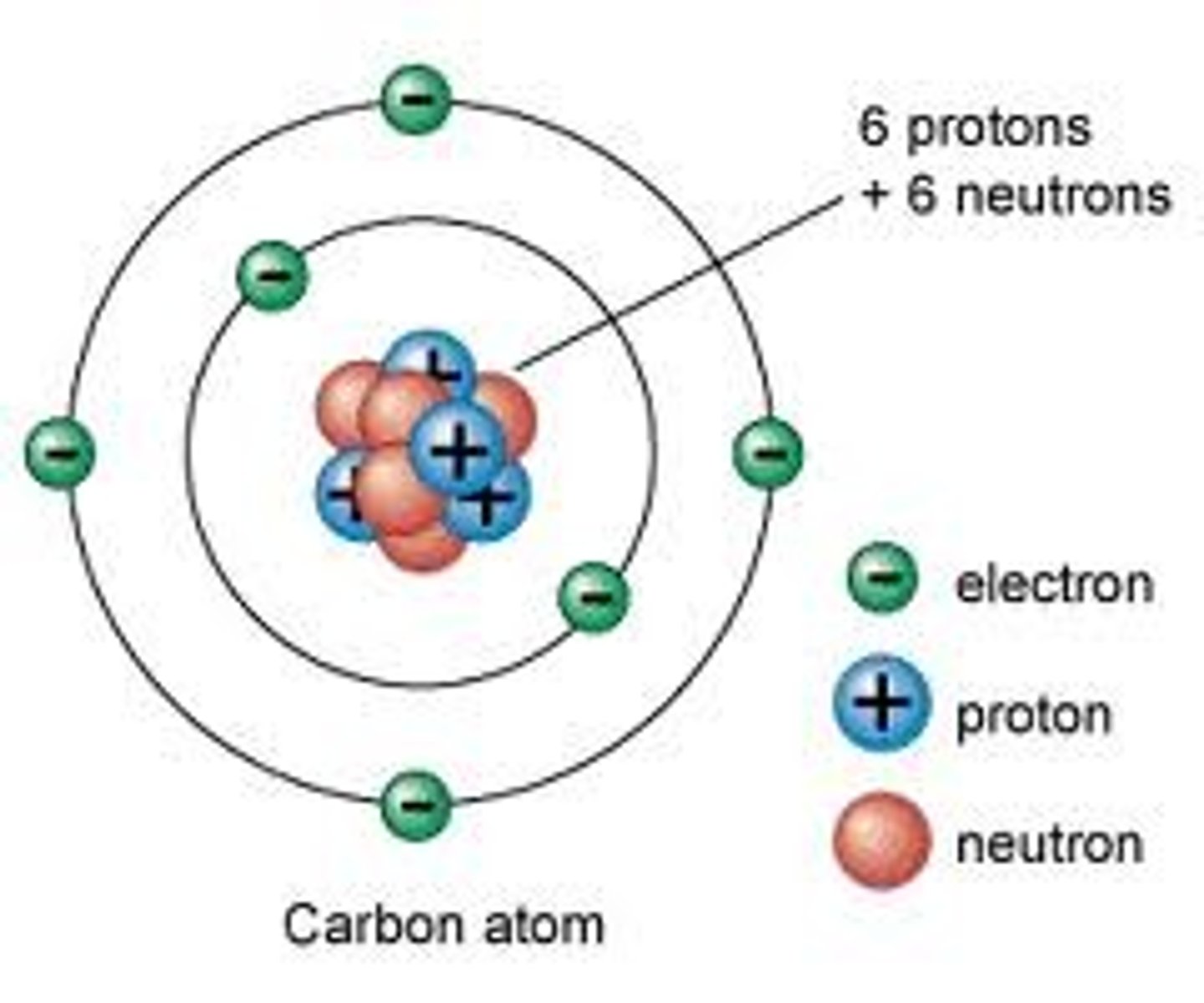
protons
positive charge, found in nucleus
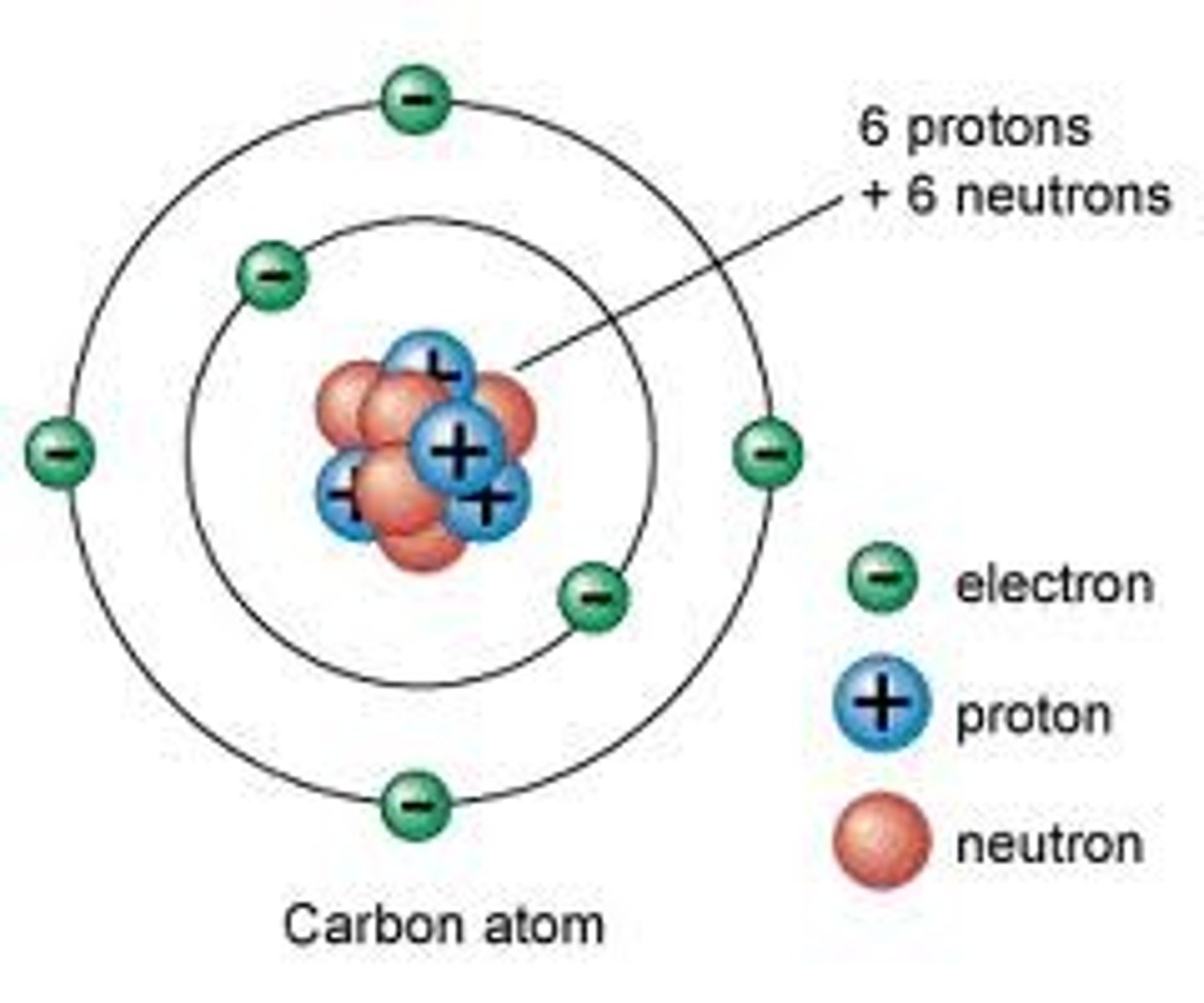
electrons
Negative charge outside the nucleus that can move
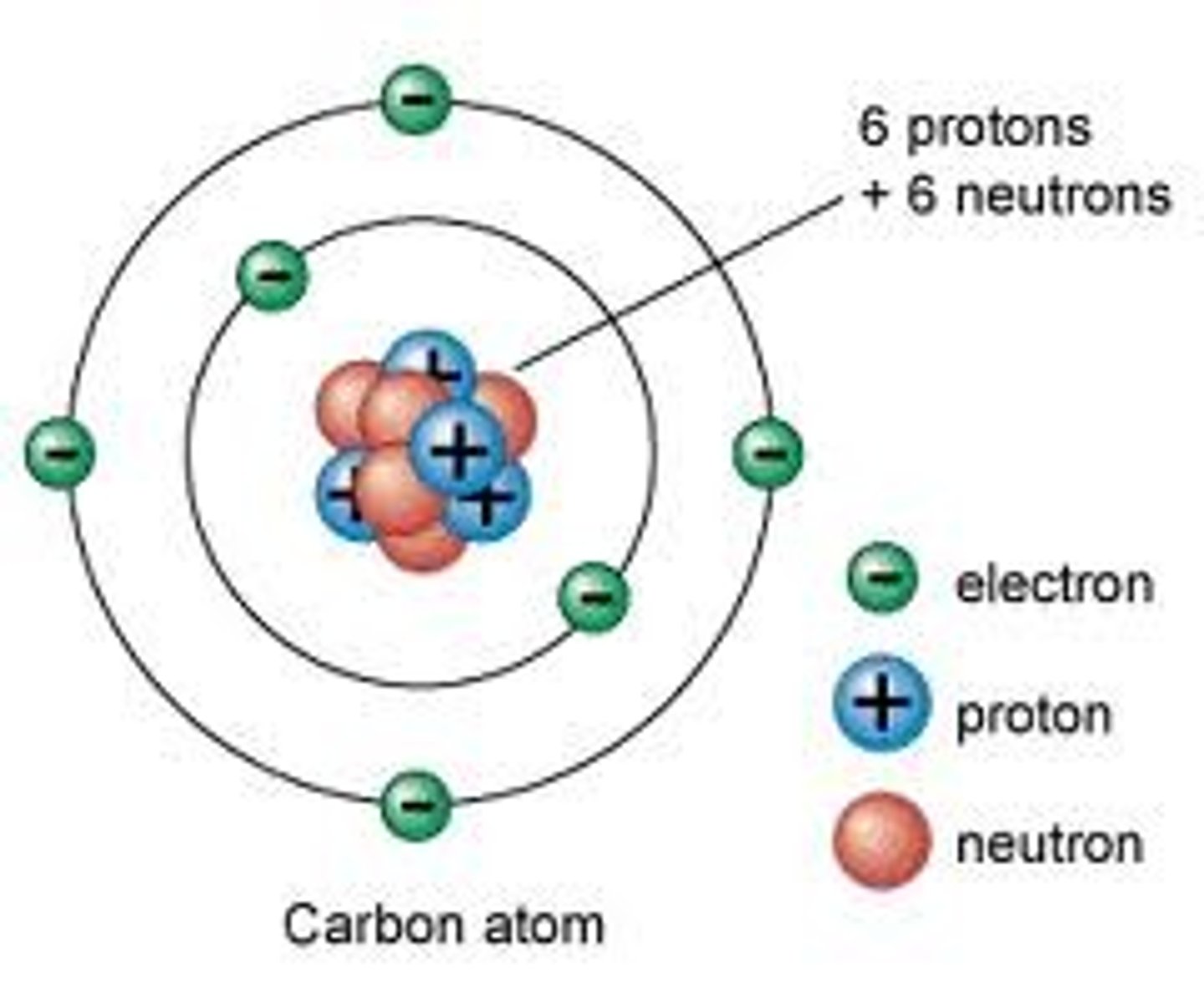
Atom
the smallest particle of an element that retains its identity in a chemical reaction
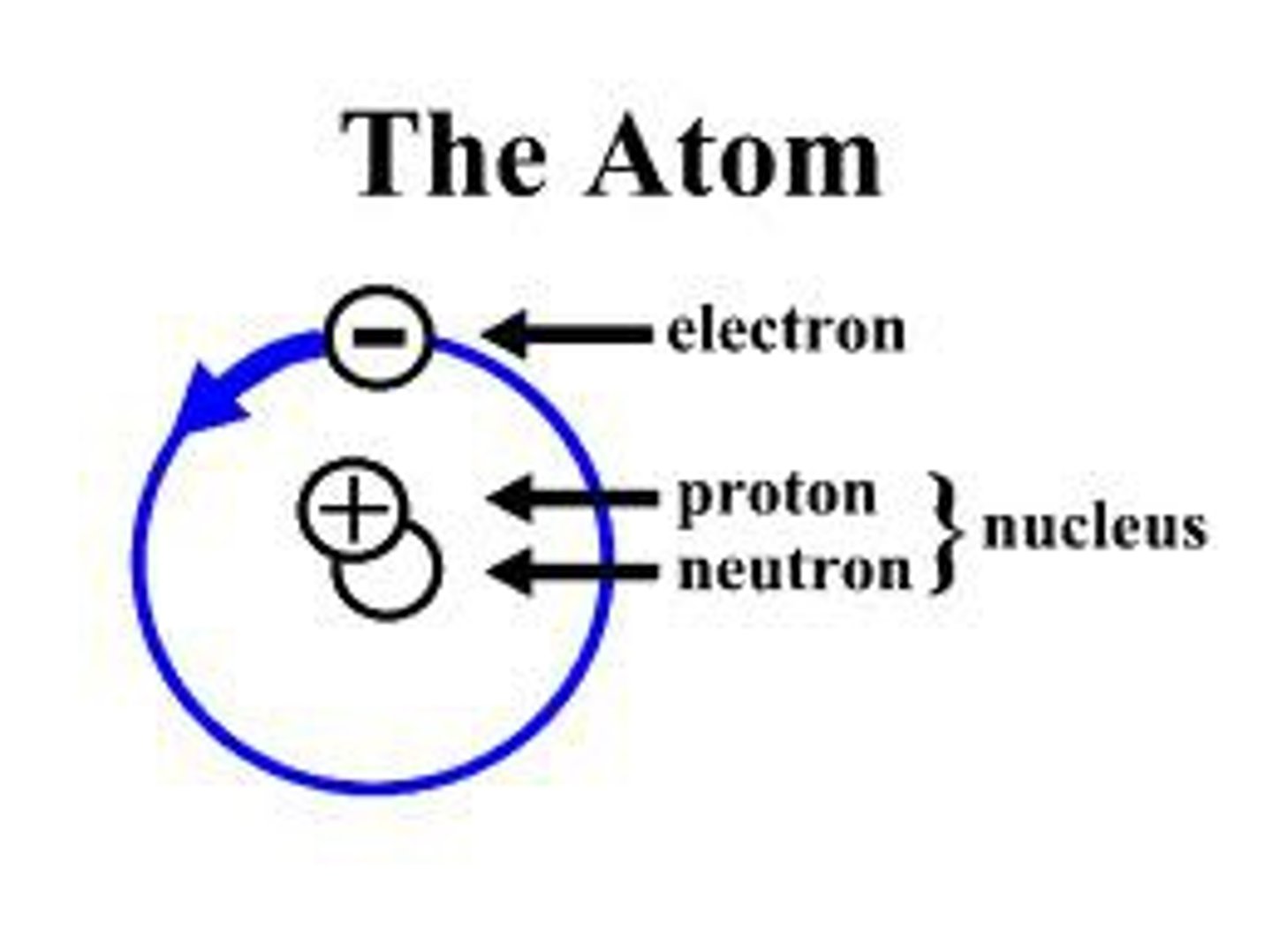
nucleus
the center of an atom, which contains the protons and neutrons; in cells, structure that contains the cell's genetic material in the form of DNA
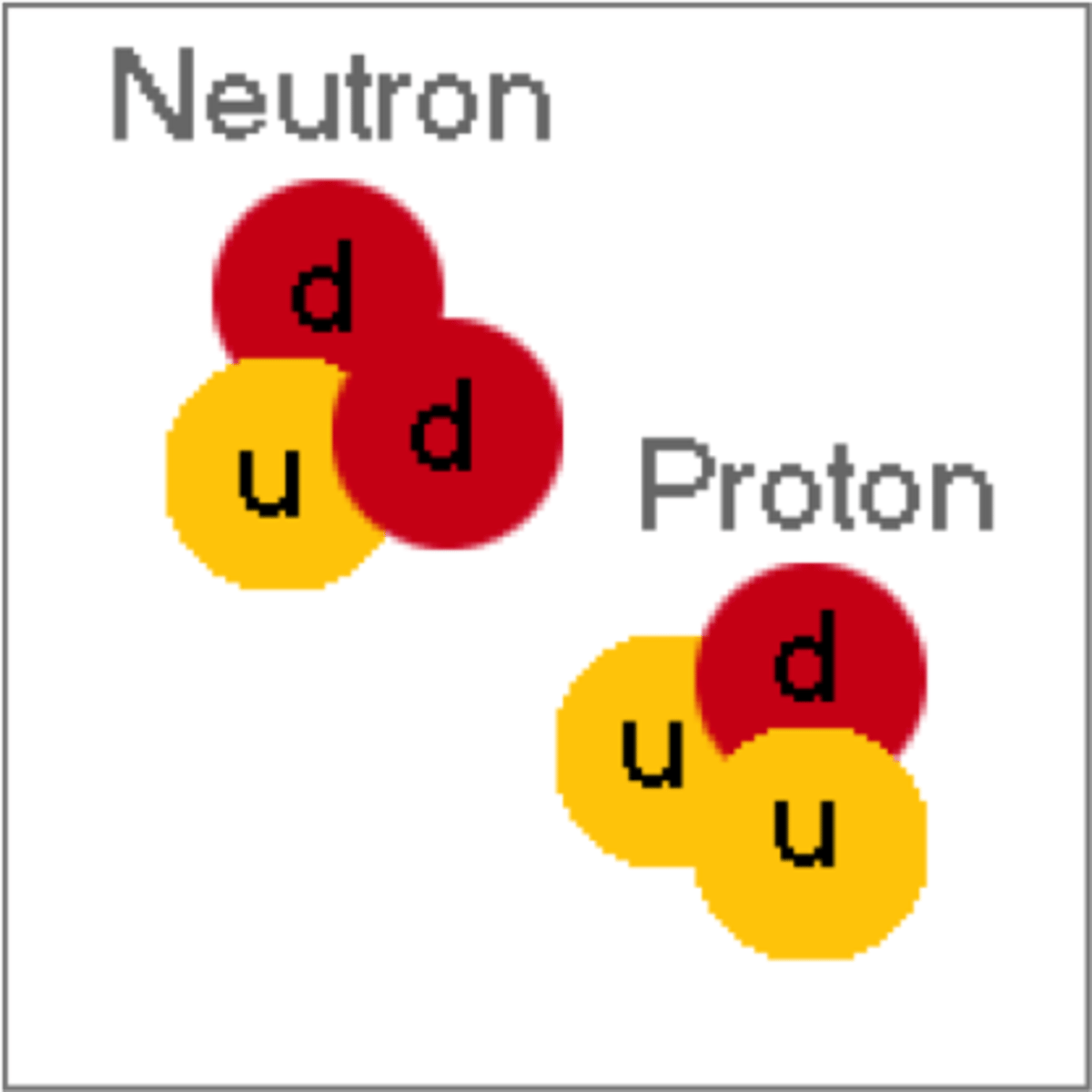
quark
particle of matter that makes up protons and neutrons
electron cloud
area around the nucleus of an atom where the atom's electrons are most likely to be found

mass number
the total number of protons and neutrons in the nucleus of an atom
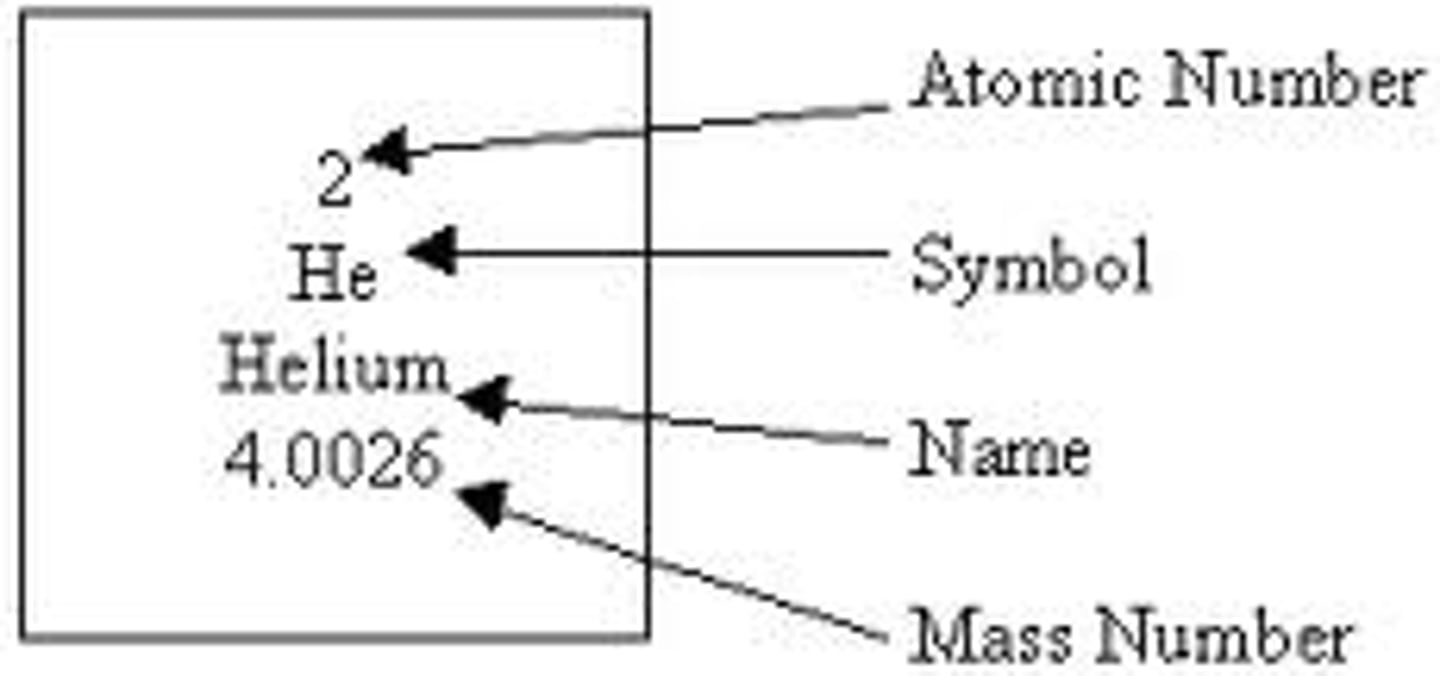
periodic table
A table that shows the elements, their atomic number, symbol, and average atomic mass; elements with similar chemical properties are grouped together.
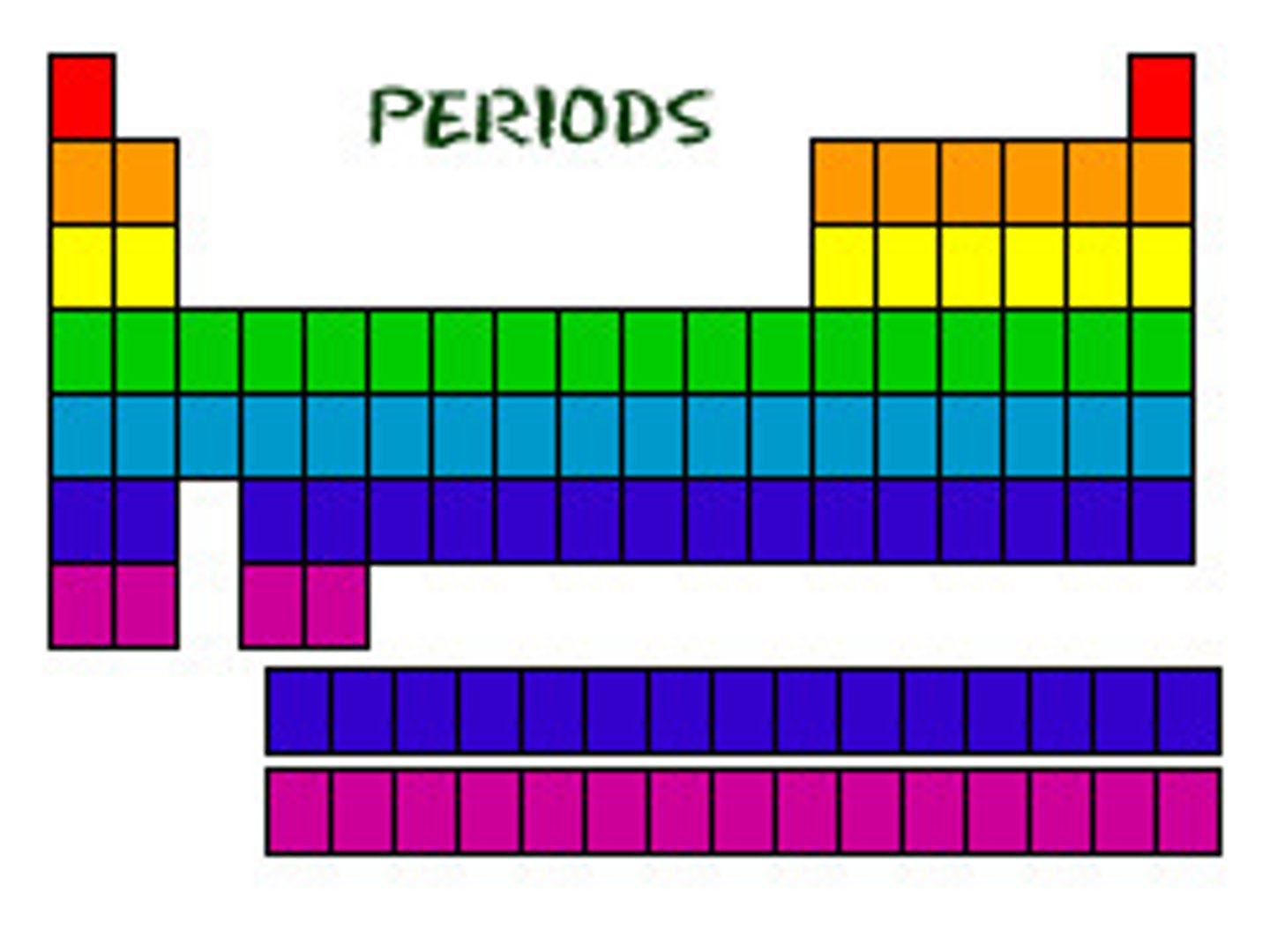
period
A horizontal row of elements in the periodic table
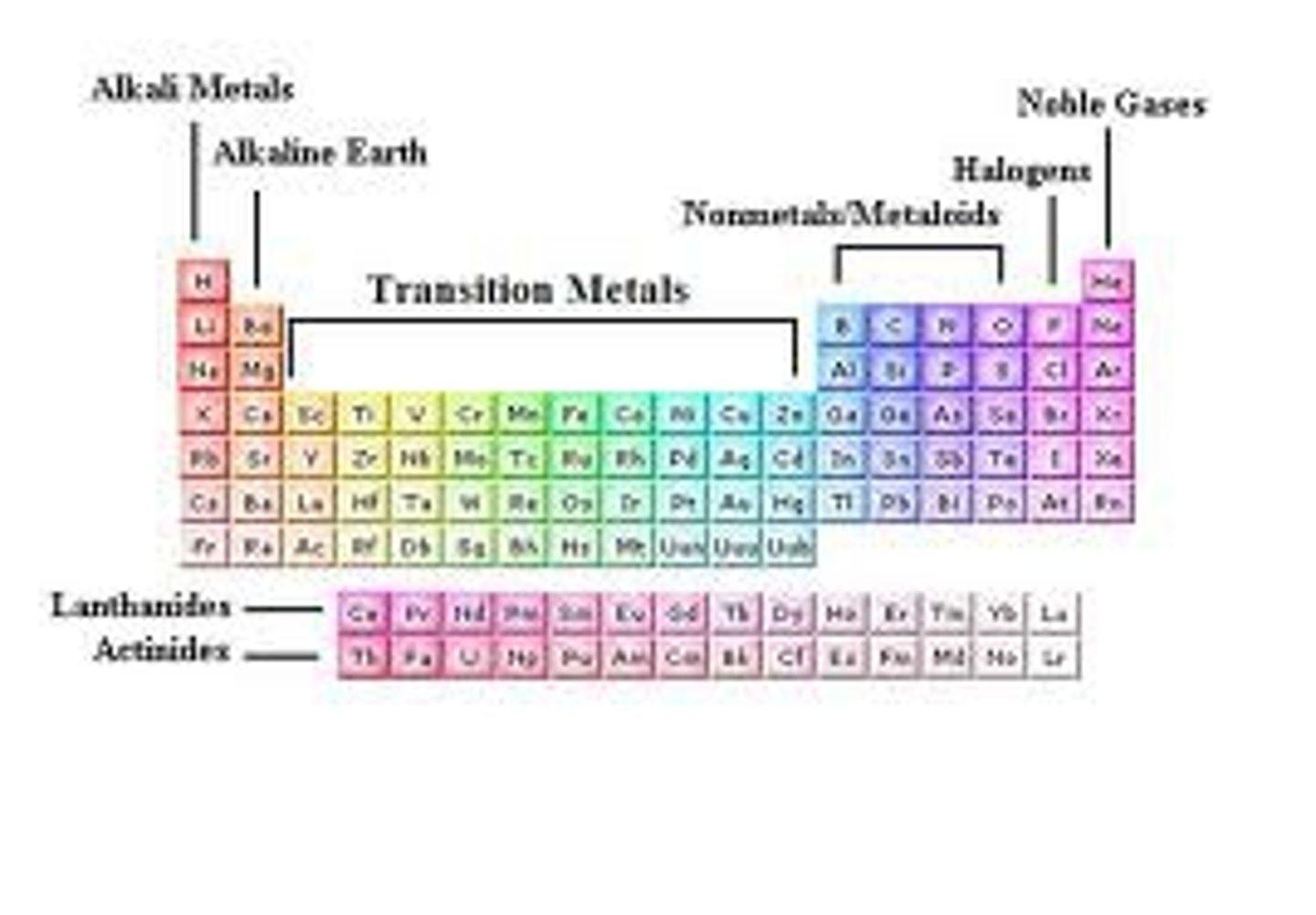
Group
a vertical column of elements in the periodic table
electron dot diagram
a model that represents valence electrons in an atom as dots around the element's chemical symbol