Chemistry Mock (electrolysis of solutions and aluminium and energy)
1/20
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
What is an exothermic reaction?
Reactions which give out heat to the surroundings.
The temperature goes up. Energy released when new bonds are made is greater than the energy absorbed to break the old bonds.
NEGATIVE TOTAL ENERGY CHANGE
What is an endothermic reaction?
Reactions which take in heat from the surroundings.
The temperature goes down. Energy released when new bonds are made is less than the energy absorbed to break the old bonds.
POSITIVE TOTAL ENERGY CHANGE
What is activation energy?
The minimum amount of energy required for a chemical reaction to occur. Ea
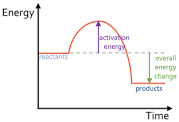
Is this exothermic or endothermic?
Exothermic
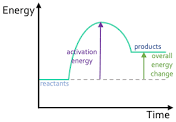
Is this exothermic or endothermic?
Endothermic
What are examples of exothermic reactions?
Combustion
Neutralisation
What are examples of endothermic reactions?
Citric acid and sodium hydrogen carbonate
Thermal decomposition
Photosynthesis
What do catalysts do?
They lower the activation energy.
This means they speed up reactions but are chemically unchanged at the end.
What is the equation for total energy change?
Bonds broken - bonds made
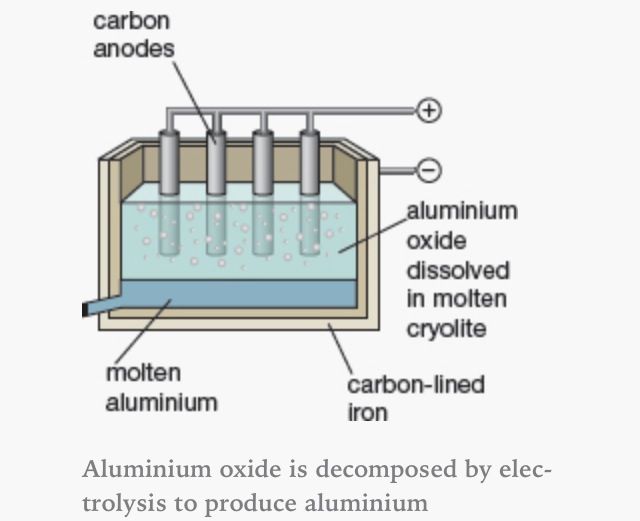
What does the diagram show?
This diagram shows using electrolysis to extract aluminium from its ore (bauxite)
What is the ionic equation at the Cathode?
(Al extraction)
Al3+ + 3e- → Al
Reduction
What is the ionic equation at the Anode?
(Al extraction)
2O2-→ O2 + 4e-
Why can’t reduction with carbon be used to extract aluminium?
Aluminium is more reactive than carbon. So it doesn’t work.
What happens to the carbon anodes?
Oxygen formed at the anode reacts with the carbon anode to make carbon dioxide which is released as a gas.This causes the anodes to break down and need replacement after 28 days.
Why is cryolite added?
To lower melting point of aluminium oxide (aluminium ore) which in turn reduces energy cost.
What takes place at the anode (positive) ?
Oxidation
What takes place at the cathode (negative) ?
Reduction
What is the cathode rule?
What is formed is determined by reactivity of metal relative to hydrogen.
Least reactive of either hydrogen or metal will form at the cathode.
(if not copper,silver,gold or platinum then it is hydrogen)
What is the anode rule?
Group 7 ions form at the anode.If no group 7 ion then OH- ions go to anode and form oxygen.
GP7 > OH-
What is the ionic equation for OH- to O2?
4OH- → O2+ 2H2O + 4e-
What is the strategy to predict products in solutions?
Is it solution?
Split the ionic compound into ions,include H+ and OH-
Use the rules to identify the product made at the electrodes
Write half equations
Identify oxidation and reduction