S3.2.2 Functional groups
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
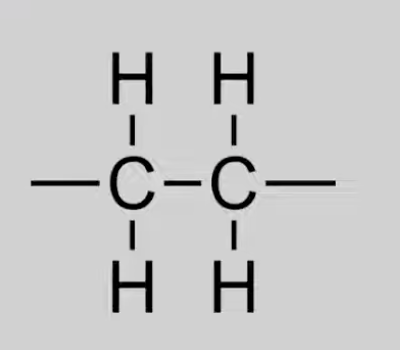
Alkyl
Saturated hydrocarbons with C–C single bonds.
General formula: CnH2n+2.
Reactions: Combustion, Free radical substitution.
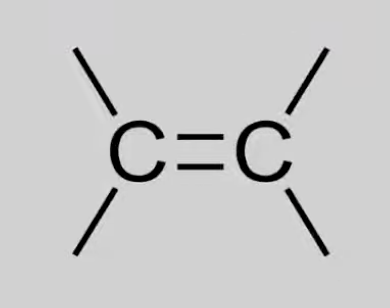
Alkenyl
Unsaturated hydrocarbons with C=C double bonds.
General formula: CnH2n.
Reactions: Combustion, Electrophilic addition, Reduction to alkanes.
Alkynyl
Unsaturated hydrocarbons with C≡C triple bonds.
General formula: CnH2n-2.
Reactions: Combustion, Electrophilic addition.
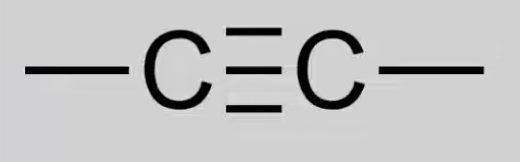
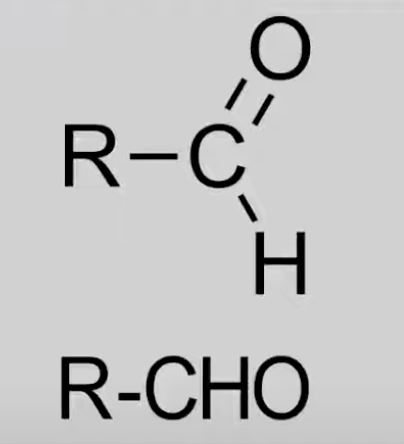
Aldehyde
Carbonyl group (C=O) bonded to H.
General formula: CnH2nO.
Reactions: Oxidation to acids, Reduction to primary alcohols (HL only).
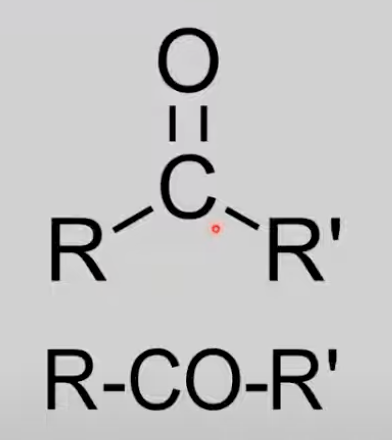
Ketones
Carbonyl group (C=O) bonded to two R groups.
General formula: CnH2nO.
Reactions: Reduction to secondary alcohols (HL only).
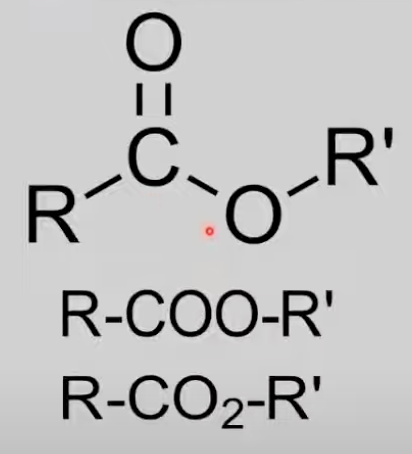
Esters
COO group between two alkyl groups.
General formula: CnH2nO2.
Formed by alcohol + acid, undergo hydrolysis.
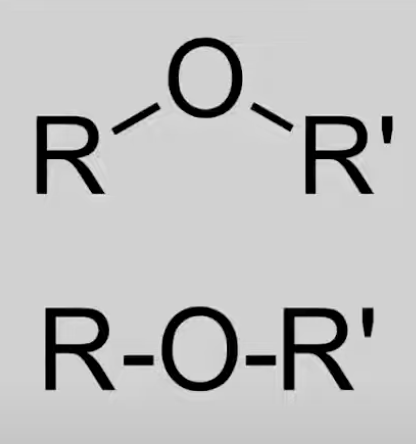
Ethers
Oxygen atom bonded to two alkyl groups.
General formula: CnH2n+2O
No reactions in IB syllabus.
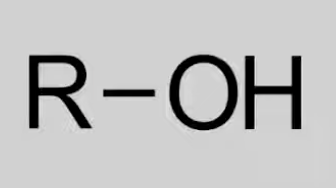
Hydroxyl
Contain -OH (hydroxy) group.
General formula: CnH2n+2O.
Reactions: Combustion, Oxidation, Esterification.
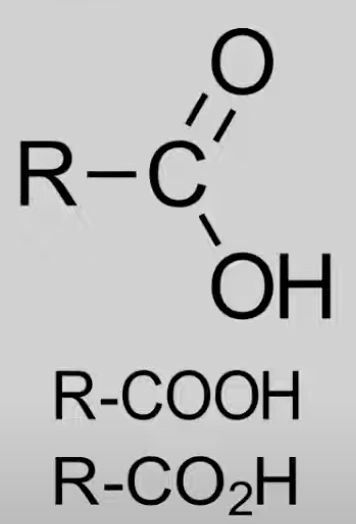
Carboxyl
Contain COOH (carboxy) group.
General formula: CnH2nO2.
Reactions: With alcohols to form esters, Reduction (HL only).
Contain C≡N triple bond.
General formula: CnH2n+1N.
Reactions not covered in IB.
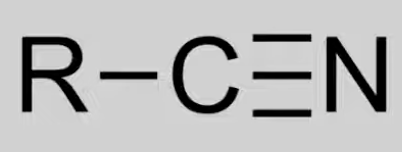
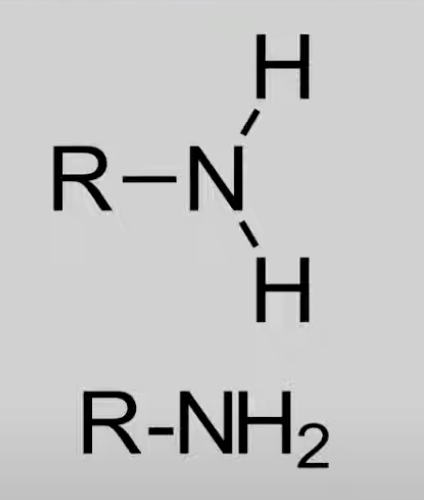
Contain -NH2 or N bonded to alkyl groups.
General formula: CnH2n+3N.
Act as Brønsted and Lewis bases.
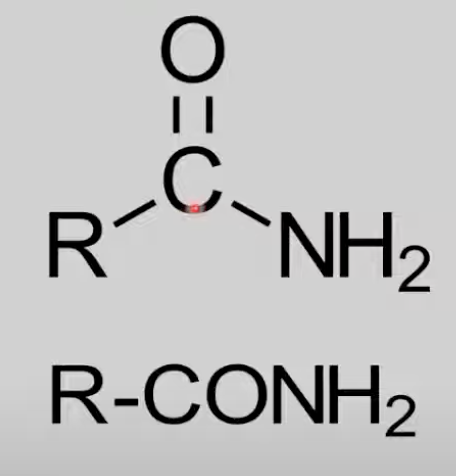
Contain CONH2 group.
General formula: CnH2n+1NO.
Reactions not covered in IB.
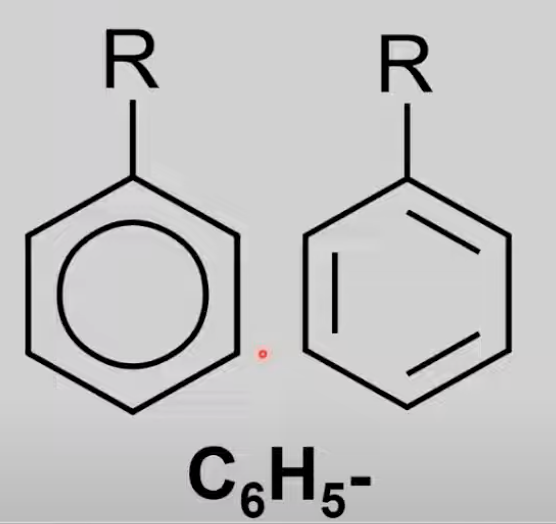
Phenyl
Aromatic compounds with phenyl group.
Benzene molecule minus a hydrogen atom
Reactions (HL): Reduction of nitrobenzene.
Alkane with halogen (F, Cl, Br, I).
General formula: CnH2n+1X.
Reactions: Nucleophilic substitution.