Silverstein and Hopper Chapter 40: Ventilator-Associated Pneumonia
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Ventilator-Associated Pneumonia (VAP)
Pneumonia that arises more than 48 hours after endotracheal intubation and mechanical ventilation that was not present at the time of intubation
What % of mechanically ventilated human patients does VAP occur in?
3-10%
Risk of Developing VAP and the Duration of Ventilation
Risk of developing VAP varies with duration of ventilation
Most animals receive mechanical ventilation for less than a week so the majority of cases of VAP would be expected to occur in the first few days of ventilation but the cumulative incidence will increase as the number of days of intubation increases
Development of VAP increases the length of time mechanical ventilation is necessary
What are the potential outcomes once a pathogen gets past the cuff of the endotracheal tube?
Pathogen may be cleared by normal respiratory defenses
The lower airways may be colonized
The tracheobronchial tree may become infected (ventilator-associated tracheobronchitis [VAT]), or if the pulmonary parenchyma becomes infected, VAP occurs
Normal Respiratory Defenses to Colonization or Infection of the Lower Airways
Cough
Mucus clearance
Humoral and cellular immune responses
How are normal respiratory defenses compromised in an anesthetized critically ill animal?
Reduced ability to cough due to sedation and presence of endotracheal tube
Inflation of a cuffed endotracheal tube depresses mucociliary clearance rate
Critical illness is associated with decreased immune system function and increased susceptibility to nosocomial infection
Evidence for neutrophil dysfunction in VAP with a reduced phagocytic capability and elevation in neutrophil proteases in the alveolar space
What is a prime risk factor for development of VAP?
Prime risk factor for development of VAP is the presence of an endotracheal tube
The risk for the development of VAP in patients receiving noninvasive mechanical ventilation is lower than in patients with endotracheal intubation
What are the two major pathologic mechanisms behind VAP?
Microaspiration past the cuff of the endotracheal tube
Biofilm development within the endotracheal tube
Pathologic Mechanisms Behind VAP - Microaspiration Past the Cuff of the Endotracheal Tube
Inflation of the endotracheal cuff allows for pooling of secretions beyond the vocal folds but above the cuff
When high-volume, low-pressure cuffs are used to prevent tracheal injury, the longitudinal folds that develop are associated with microaspiration or macroaspiration of subepiglottic fluid with subsequent translocation of bacterial to the interior of the endotracheal tube or airways
Pathologic Mechanisms Behind VAP - Biofilm Development within the Endotracheal Tube
Once bacteria are present on the internal surface of the endotracheal tube, these bacteria may easily adhere and produce a biofilm
The biofilm in inaccessible to antimicrobials unless they are aerosolized
As bacteria proliferate within the endotracheal tube, they may be dislodged into the lower airway because of airflow, suctioning, or bronchoscopic procedures
Exogenous Sources of Bacteria Associated with Biofilm Formation of VAP
Contaminated respiratory equipment
The environment
Healthcare provider’s hands
Endogenous Sources of Bacteria Associated with Biofilm Formation of VAP
Endogenous bacteria
Normal oral flora is typically a mixed population of bacteria
In critical illness aerobic Gram-negative bacteria predominate
Change in type and increased numbers of bacteria attributed to lack of oral hygiene seen with normal swallowing that results in the spread of saliva which contains proteases, immunoglobulins, and enzymes
Antimicrobials can also change the population and increase resistance of oral flora
Gastric bacteria
Critically ill patients receiving gastric antacid medications show greater rates of gastric colonization than those who do not
Enteral feeding has been associated with the development of VAP in humans
What type of bacteria represent the majority of VAP cases?
Aerobic
Ventilator-Associated Condition
At least 2 days of worsening oxygenation following at least 2 days of stability on the ventilator based on changes to the fraction of inspired oxygen or positive end-expiratory pressure
Include all forms of ventilator induced lung injury as well as infection-related ventilator-associated conditions (IVACs)

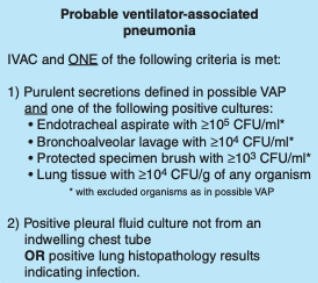
Infection-Related Ventilator-Associated Condition (IVAC)
IVACs are diagnosed based on elevated or reduced body temperature, leukocytosis, or leukopenia, and a new antimicrobial agent that is started and continued for 4 or more days
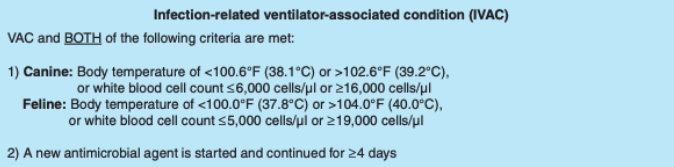
Possible Ventilator-Associated Pneumonia
Possible VAP can be diagnosed after an IVAC has been diagnosed - when purulent secretions from lungs, bronchi, or trachea contain 25 or more neutrophils and 10 or less squamous epithelial cells per low power field or a positive culture of sputum, endotracheal aspirate, bronchoalveolar lavage, lung tissue, or protected specimen brushing is found
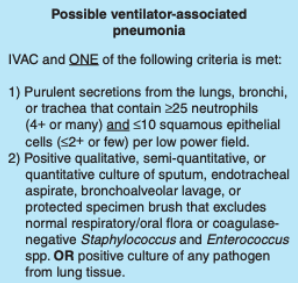
Probable Ventilator Associated Pneumonia
Probable VAP can be diagnosed when either purulent respiratory secretions are found (as stated in possible VAP) and a positive quantitative/semi-quantitative culture is found, or a positive pleural fluid culture is obtained not from an indwelling chest tube or lung histopathology
Modified Centers for Disease Control and Prevention Surveillance Definitions for Ventilator-Associated Pneumonia (PNU1)
Imaging Test Evidence (Two or More Serial Chest Imaging Test Results with at Least One of the Following)
New and persistent or progressive and persistent
Infiltrate
Consolidation
Cavitation
Signs/Symptoms
At least one of the following
Fever
Leukopenia or leukocytosis
And at least one of the following
New onset or change to purulent sputum or increased respiratory secretions
New onset or worsening cough or respiratory distress
Evidence of worsening gas exchange (e.g. oxygen desaturation, increased oxygen requirements, or increased ventilator demands)
Crackles or bronchial breath sounds
Laboratory
At least one of the following
Organism identified from blood, pleural fluid, or lung tissue culture
Positive culture or 5% or more cells with intracellular bacteria from lower respiratory tract
Appropriate histopathologic evidence
Patients must fulfill all three (Imaging, Signs/Symptoms, and Laboratory) criteria
VAT
VAT is an infection of the tracheobronchial tree of similar origin to VAP but does not affect the pulmonary parenchyma
May produce the same clinical signs as VAP
What is indicated if the patient fulfills the criteria for VAT/VAP?
Thoracic imaging
A new or progressive pulmonary infiltrate on thoracic imaging is required to diagnose VAP
Limitations for Airway Sampling for Microbial Culture
Endotracheal aspirates may represent colonization of the endotracheal tube rather than true infections
If a nonbronchoscopic techinque is used, fluid from a noninfected part of the lung may be sampled
May take up to 48-72 hours for culture results to return
Nonpharmacologic Strategies for Prevention of VAP
Provide educational program for caregivers and monitoring of compliance
Use of strict alcohol-based hand hygiene
Minimize time of intubation with weaning protocols
Do not change ventilatory circuit unless contamination occurs
Aspiration of subglottic secretions
Maintain endotracheal tube cuff pressure at 25 cmH2O or more
Minimize nurse to patient Ratio
Pharmacologic Strategies for Prevention of VAP
Perform oral care with dilute chlorhexidine
Avoid increasing gastric pH prophylactically
Favorable effects seen with 0.12-2% chlorhexidine
Performing oral antisepsis 2-4 times a day recommended
Routine use of gastric antacid drugs is not recommended in any species on mechanical ventilation
Reserve for use in cases of demonstrated gastrointestinal ulceration
Treatment for VAP
Initiation of antimicrobial therapy for VAP should commence as soon as there is clinical suspicion and airway microbiological samples have been taken and analyzed
Risk Factors for MDR Organisms in VAP
Prior antimicrobial use within 90 days
Septic shock at the time of diagnosis
Acute respiratory distress syndrome preceding VAP
Hospitalization for 5 or more days prior to occurrence of VAP
Antibiotics for Treatment of VAP
Use of an antipseudomonal antimicrobial empirically such as a fluoroquinolone, piperacillin-tazobactam, or ceftazidime for VAP is recommended due to its high prevalence in this disease
Carbapenems are a reasonable empiric option for dogs and cats when risk factors for MDR organisms are present
IV aminoglycosides aren't used as monotherapy because of their poor penetration into infected lung tissue
Aerosolized Antimicrobials for Treatment of VAP
Aerosolized antimicrobials have the potential advantage of achieving high drug concentrations in the lungs and potentially reaching biofilms while avoiding systemic absorption and toxicities
Aminoglycosides or polymyxins most commonly used
Both have concerns for nephrotoxicity
Use an ultrasonic or vibrating plate nebulizer with mechanical ventilation to maximize delivery to site of infection
Current recommendations are to include inhaled antimicrobials when VAP is due to Gram-negative bacilli (e.g. Acinetobacter spp or Pseudomonas aeruginosa) that are susceptible to only aminoglycoside or polymyxins
How long should the course of antimicrobials for treatment of VAP be?
Majority of infections can be treated by a course of appropriate antimicrobials for a total duration of 7 days
If a fermenting Gram-negative bacillus is cultured, a 14- to 21-day course of antimicrobial therapy should be considered