C1 Enzymes and Metabolism, Cell Respiration, Photosynthesis
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
Enzymes
Biological catalysts made by living organisms. Cause very large increases in the rates of chemical reactions in cells without being used themselves.
Major Chemical Conversions
Happen via a series of reactions catalyzed by different enzymes. The product of one enzyme’s reaction is the substrate for the next enzyme.
Interdependence
When the activity of both being restricted if one is not active enough.
Metabolism
The complex network of interdependent and interacting chemical reactions catalyzed by enzymes that occur in living organisms.
Anabolism
The energy-requiring part of metabolism in which simpler substances are transformed into more complex molecules
Photosynthesis Reactions
Anabolic reactions that produce sugars and other carbon compounds from carbon dioxide and water with light as the energy source.
Catabolism
The energy-releasing part of metabolism where complex molecules are broken down into simpler ones, producing energy.
Cell Respiration Reactions
Catabolic reactions in which glucose or fats are oxidized to release energy, with water and carbon dioxide as waste products.
Active site
Site on surface of an enzyme where catalysis occurs. May only consist of a few amino acids, three dimensional shape, chemical properties that cause substrates to rapidly change into products.
Collision
The meeting of a substrate and active site. The shape and chemical properties of the active site complement those of the substrate, so when close enough and in appropriate orientation the two bind together.
Induced-Fit Binding
The concept of substrate and active site achieving complementarity through mutually induced changes.
Factors Affecting Enzyme Activity
Temperature
Substrate-concentration
pH
Enzymes and Heat Relationship
Enzyme activity increases with heat because collisions between the substrate and the active site are more frequent due to faster molecular motion.
Enzyme Heat Denaturation
At high temps enzymes are denatured because heat causes vibrations which break bonds needed to maintain the structure.
Enzyme-Substrate Concentration Relationship
As substrate concentration increases more and more of the enzymes active sites are occupied.
Enzyme and pH Relationship
Enzymes have an optimum pH where enzyme activity is the fastest. PH 7 is optimum for most enzymes.
Enzyme and pH Denaturation
pH affects the ionization of COOH and NH2 groups, which alters enzyme conformations and can cause permanent denaturation.
Activation Energy
The amount of energy needed to break bonds within the reactant and to reach the transition state. Enzymes reduce this by weakening bonds.
Exothermic Reaction
A chemical reaction that releases energy, usually in the form of heat, as the reactants transform into products.
Endothermic Reaction
A chemical reaction that absorbs energy from its surroundings, usually in the form of heat, resulting in a temperature decrease in the environment as reactants are converted into products.
Properties of ATP
Chemically stable at neutral pH levels typical of cells
Soluble in water so ATP can diffuse freely in cytoplasm
Unable to diffuse through the phospholipid bilayers of membranes
Can release a quantities of energy that can be used for cellular processes and reactions.
Can be easily regenerated by adding a third phosphate group to ADP
Converting between ATP and ADP
Energy has to be invested, coming from sources like sunlight, oxidation of food, or a transfer of another phosphate group from other compounds.
Reactions to convert from ATP to ADP and vice versa
ADP → ATP iS a phosphorylation and condensation reaction
ATP → ADP is a hydrolysis reaction
Aerobic Cell Respiration Characteristics
Uses oxygen
Can use the substrates sugars and lipids
Produces 30-32 ATP per glucose (a large yield)
Waste products are CO2 and H2O
The reactions occur in the cytoplasm and mitochondria
Aerobic Cell Respiration Reaction
glucose + oxygen → carbon dioxide + water + energy
Anaerobic Cell Respiration Characteristics
No oxygen
Uses the substrates glucose and sugars
Produces only 2 ATP per glucose (a small yield)
The waste products are lactate (lactic acid)
Only happens in cytoplasm
Anaerobic Respiration Reaction
glucose → lactate + energy
Respirometers
Devices used to measure the rate of cell respiration.
A sample is put into the apparatus, where it can absorb oxygen from and excrete CO2 into the air around it. It measures the air pressure/volume of the air inside.
Temperature changes affect air pressure so must prevent it from heating up or cooling down.
Photosynthesis
How light energy is transformed into chemical energy when absorbed by pigments (coloured substances such as chlorophyll)
Photosynthesis Formula
Carbon dioxide + water = glucose + oxygen
6CO2 + 6H2O = C6 H12 O6 + 6O2
Requires solar energy
Organisms that photosynthesize
Plants, Algae, Cyanobacteria.
They use some of the oxygen produced in aerobic cellular respiration (ie. roots of plants) But most is excreted into the environment.
In underwater organisms the oxygen emerges as bubbles.
Process of Chromatography
Tear leaf into small pieces
Grind leaf with pestle and mortar and sharp sand and propanone to extract pigments
Transfer sample of extract into watch glass
Evaporate until dry with hot air from hair dryer
Add a few drops of propanone to dissolve the pigments
Transfer drops of the solution to form a concentrated spot 10mm from the end of a strip of chromatography paper
Suspend strip in a tube with base dripping into a running solvent
Remove the strip from the tube when running solvent has nearly reached the top
The pigment in each spot can be identified by its Rf value
Rf = distance moved by spot/distance moved by solvent
Wavelengths of Light
400nm to 700nm reach the earth’s surface and this range is used both in photosynthesis and human vision.
Violet is shortest wavelength and red is the longest. The shorter the wavelength, the more energy a photon of light has.
The absorption spectrum for the 2 most common types of chlorophyll
Chlorophyll absorbed red and blue light the most effectively. Very little green light is absorbed, mostly reflected, making it appear green to us.
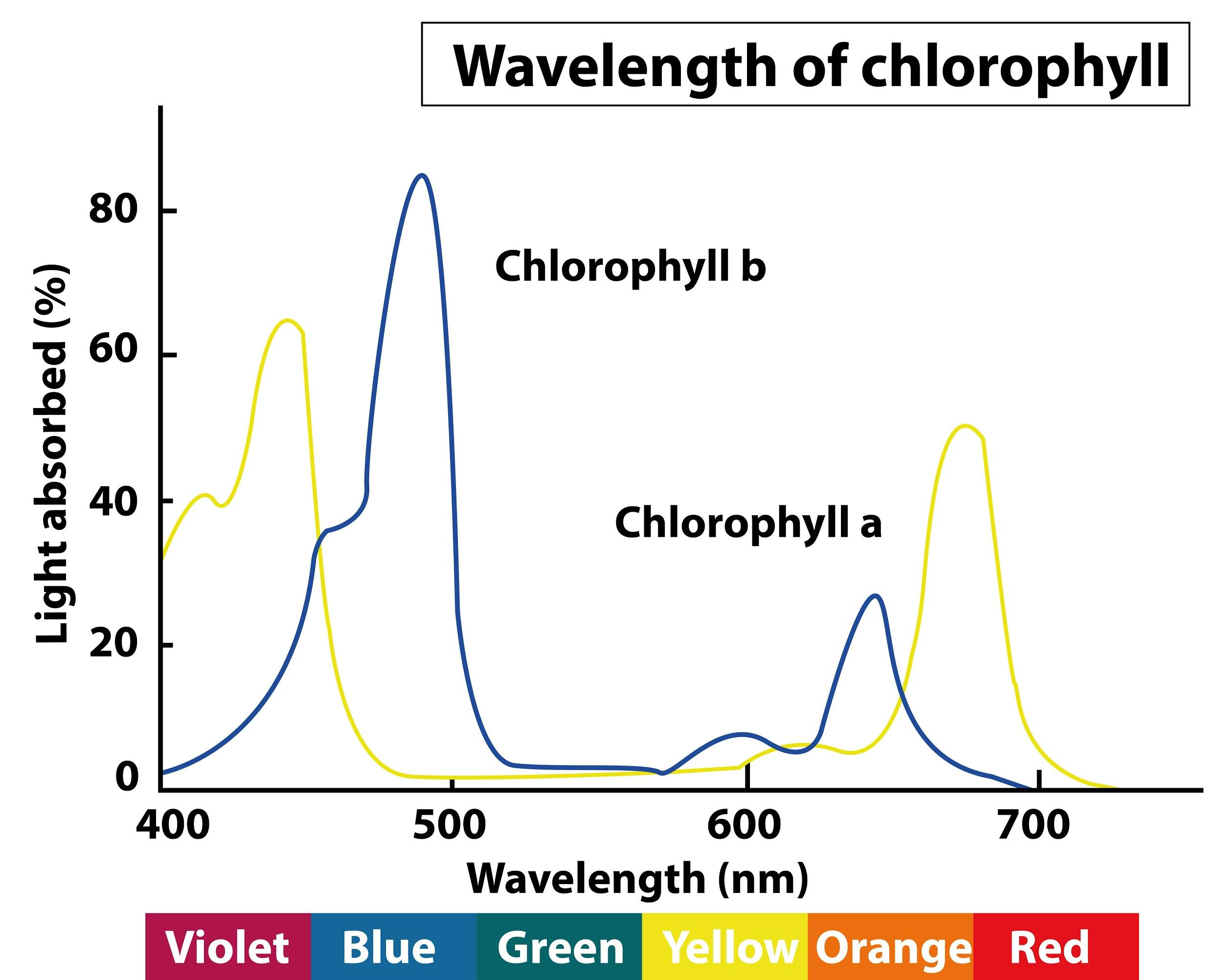
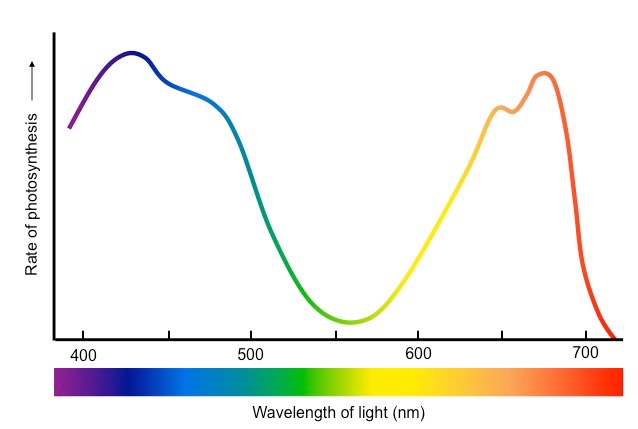
Action Spectrum
Similar to Absorption Spectrum with peak in blue and red and trough in green. Some green light is still used in photosynthesis due to accessory pigments.
Limiting factors for rate of photosynthesis
Temperature
Light intensity
Carbon dioxide concentration
Carbon Dioxide Enrichment Experiments
Large-scale studies that expose vegetation to elevated CO2 concentrations under natural conditions. Allows researchers to study the effects of increased CO2 on plants. Help scientists understand how rising atmospheric CO2 levels might impact ecosystems.