Unit 3: Biosignaling
1/132
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
133 Terms
transduction
converts an extracellular signal to an intracellular response
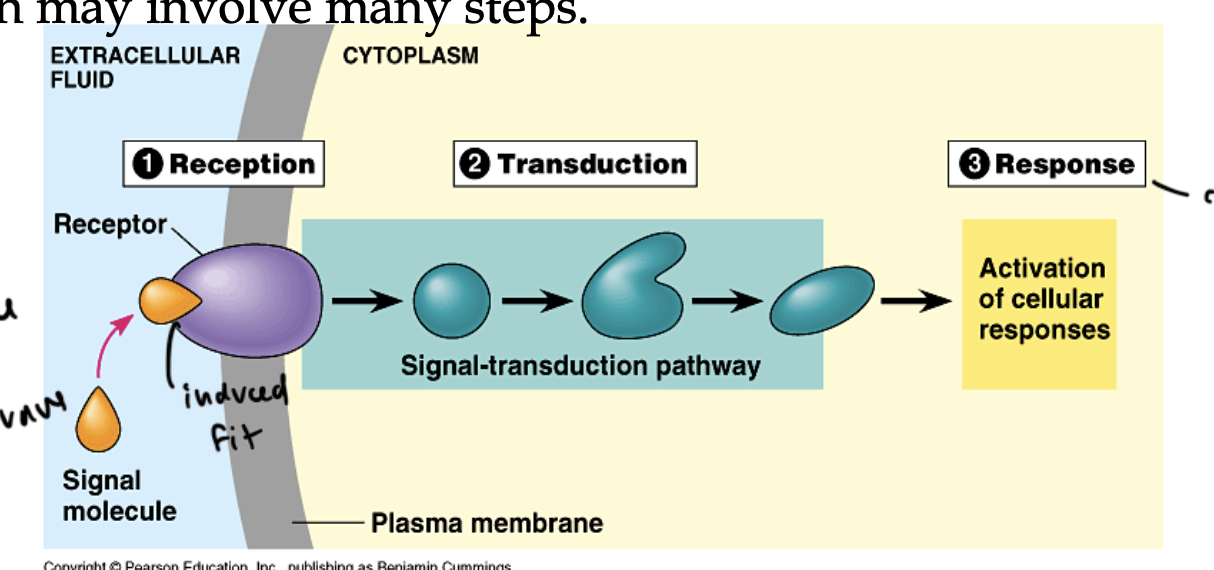
where can you find receptors
free floating in cytosol
nucleus
at cell membranes
ligand-gated channels are in either
open or close conformation
specificity
how many different ligands can bind to your receptor
sensitivity
how well the ligand binds to the receptor
you want a low Kd
avidity
summing responses using multiple binding sites, so that when one site lets go of the ligand, the next site binds to it
amplification
when each enzyme at a step activates many more enzymes
modularity
idea that one signal can cause multiple separate responses
integration
when different signals can form a combined response or cancel each other out
“crosstalk”
3 parts of G-proteins
alpha, gamma, beta
G-proteins bind guanine di or triphosphate (GDP or GTP)
Gs: stimulatory
Gi: inhibitory
glycogen breaks down into
glucose
3 types of biosignals
autocrine
paracrine
endocrine
4 major types of biosignaling systems
GPCR
receptor tyrosine kinase (and kinase cascades)
gated ion channel
nuclear receptor
signal transduction/cell signaling definition
the transmission of molecular signals from the environment that are not membrane permeable to the interior of the cell
things that cells can respond to
light
mechanical touch
neurotransmitters
nutrients
odorants
tastants
antigens
growth factors
hormones
glycoproteins/oligosaccharides
developmental signals
ECM components
changes that cell signals can evoke
differentiation and antibody production
growth in size, shape, or strength
gene expression
ability to divide (sexual or asexual reproduction)
autocrine signaling
acts on the cell the produced the signal
paracrine signaling
localized signaling to a nearby cell
endocrine signaling
distant signaling through the bloodstream using hormones
the binding of a signal to the receptor causes
the receptor to change in some way, usually a shape change (induced fit occurs)
this initiates a downstream response
if a receptor is free floating, it must be able to
get to the plasma membrane on its own
examples of each main type of receptor
GPCR: epinephrine receptor
enzyme-linked: insulin receptor
ligand-gated: nicotinic ACh receptors
nuclear: steroid receptors
other membrane: integrin receptors (for cell crawling/attachment)
the human genome has _____ GPCRs and we have _____ drugs that target them
>800
>700 drugs to target them
20% of all cancers are due to a mutation in
GPCR genes
about ____% of drugs target GPCRs
25
GPCR drugs can treat
hypertension, drug abuse, mental health, asthma, etc
types of receptors often targeted by drugs
GPCR
enzyme-linked
ion channels
others
desensitization
turning off a pathway
integration
when two signals have opposite or summing effects to create a response
divergence
when a receptor gets activated and activates two different pathways taht have different end effects
localized responseq
when the enzyme that destroys an intracellular message is clustered with the message producer, so that the message is degraded before it can diffuse to distant targets, creating a brief and local response only
typical ligands
small ions
organic molecules
polysaccharides
peptides
proteins
what Kd values show a high affinity of receptors for their signal molecule/
10^-7 or less
cooperativity
how well binding at one site influences binding at another site
clutsering
can use lipid rafts or maybe things are just in close proximity to speed up rxns
avidity
when one receptor releases its ligand and the next immediately binds that same ligand, combining affinity for an immediate response
every cell expresses different proteins, so
they respond to the same signal in different ways
crosstalk
integration of multiple signals
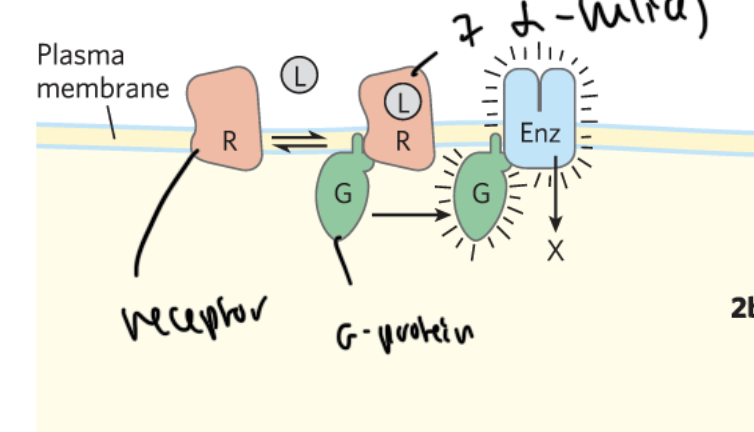
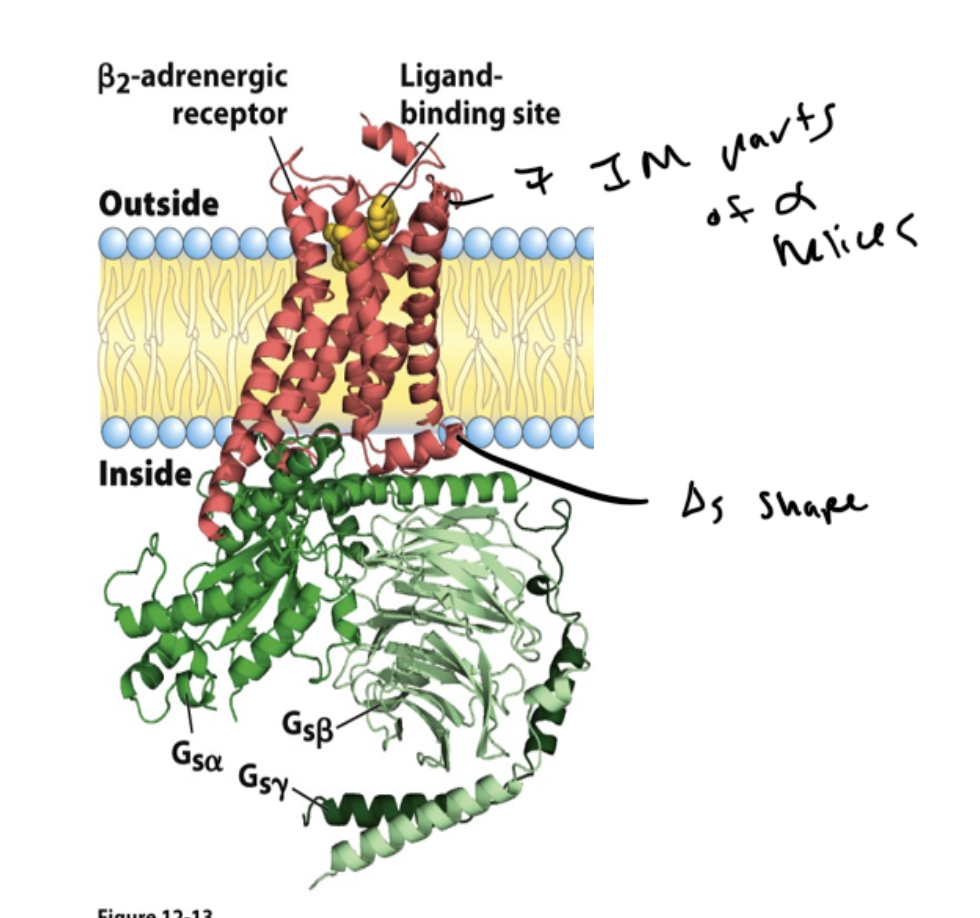
parts of a GPCR
receptor is transmembrane and has 7 alpha helices through it
ligand binds to the receptor
this activates a G-protein, which regulates an enzyme
receptor tyrosine kinase
ligand binding activates a tyrosine kinase by autophosphorylation, causing a kinase cascade, eventually altering gene expression
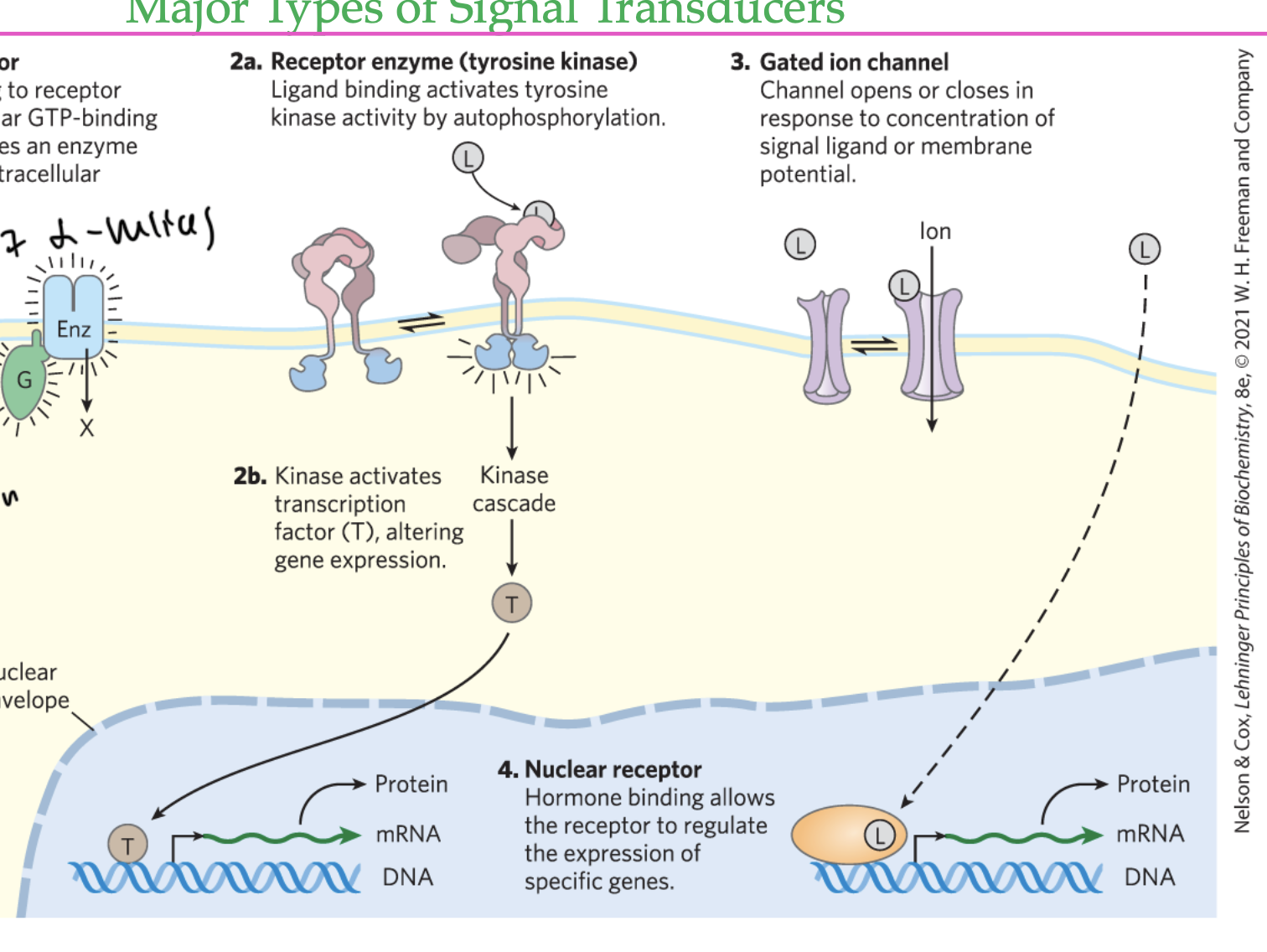
gated ion channel
ligand goes through the channel and binds to a protein, altering gene expression
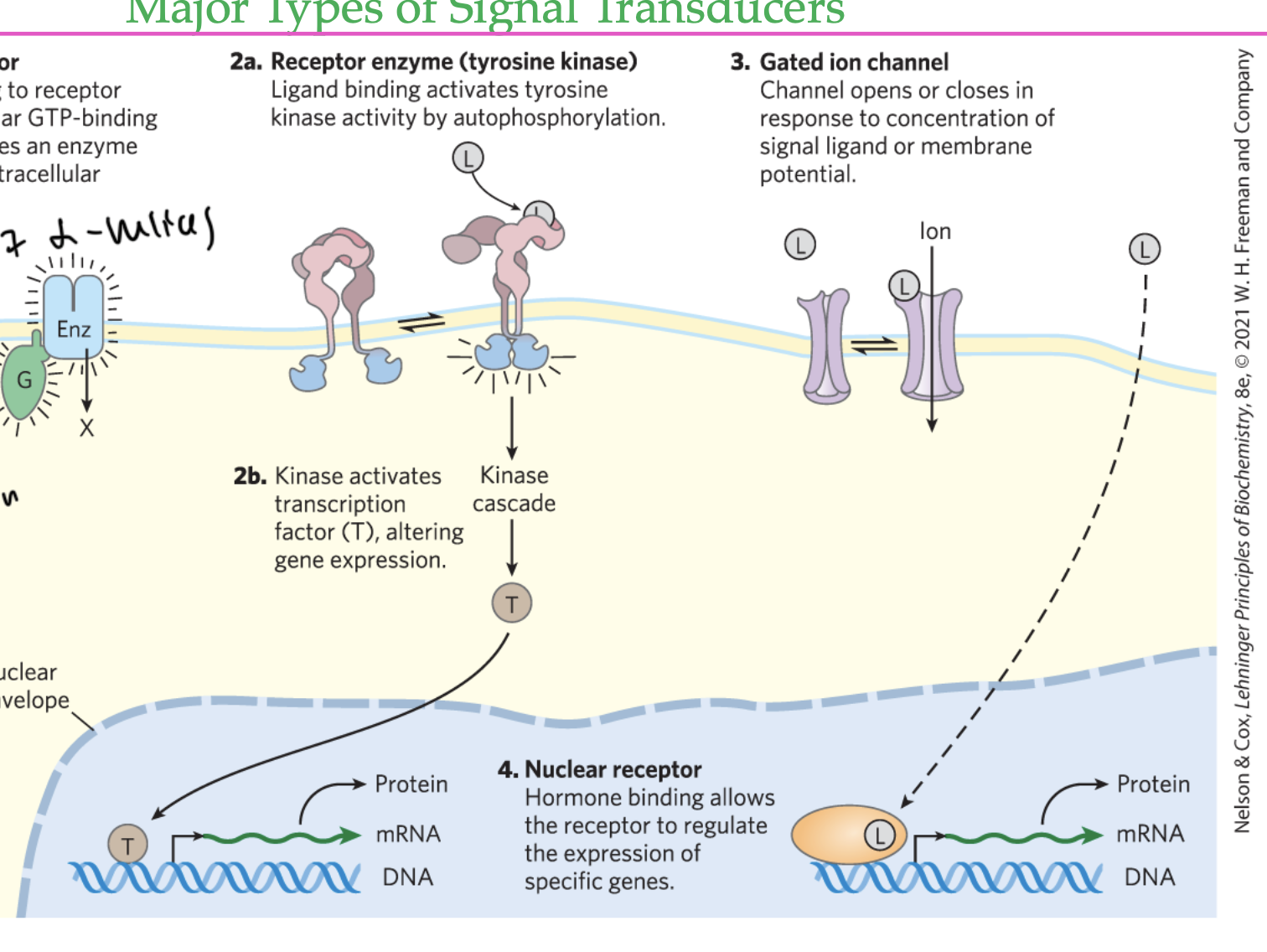
GPCRs are _____
heterotrimeric membrane-associated proteins
have alpha, gamma, and beta subunits
the receptor changes shape when the ligand binds
epinephrine
aka adrenaline
made in the adrenal glands on top of the kidneys and is secreted thru the bloodstream as an endocrine signal
mediates the stress/sympathetic response
binds to receptors in muscle and liver cells to initiate breakdown of glycogen for energy
also binds to receptors in adipose cells to induce lipid hydrolysis
also binds receptors in heart cells to incr HR
which subunits of the G-protein in a GPCR are lipid anchored?
gamma and alpha
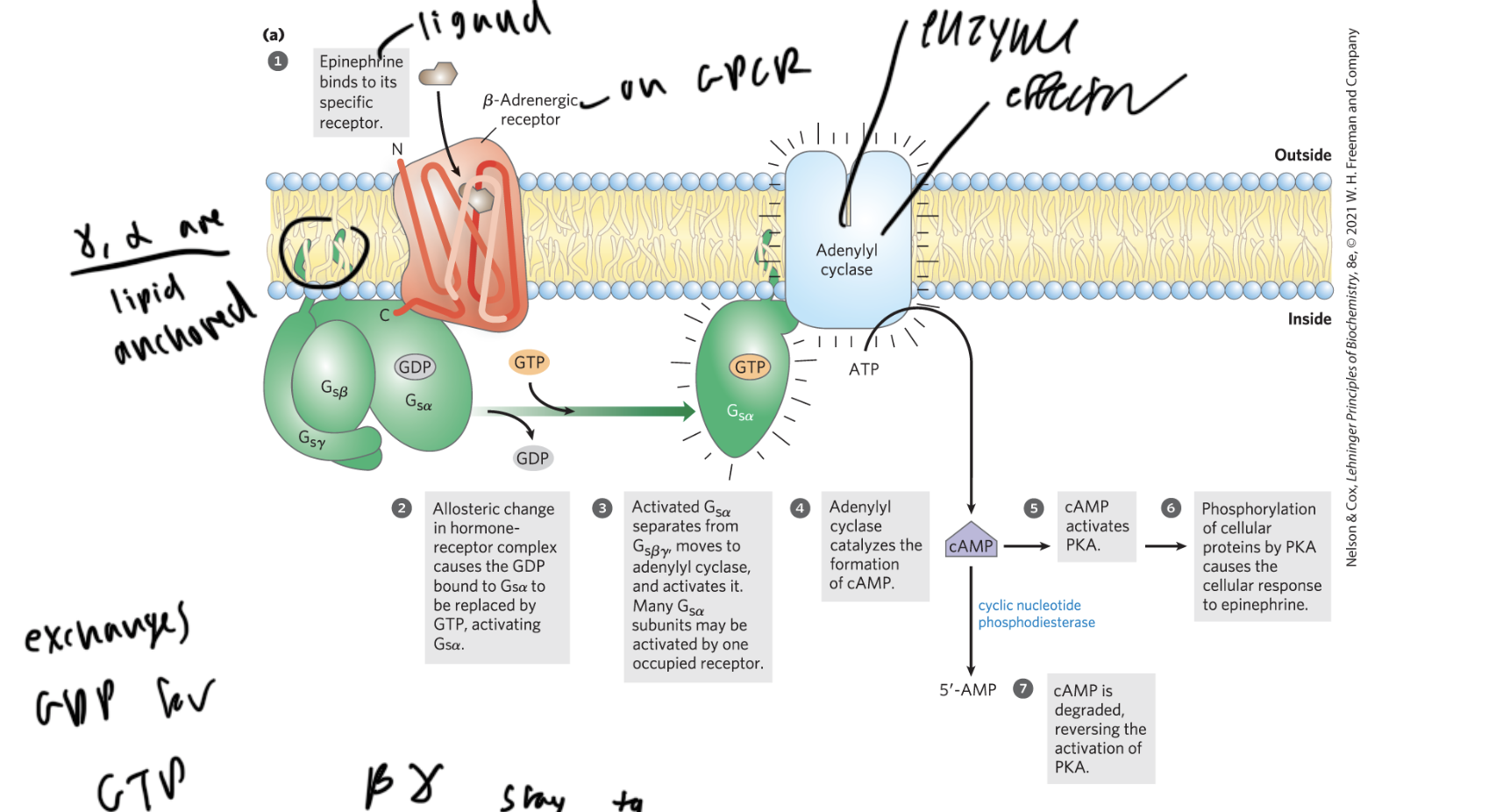
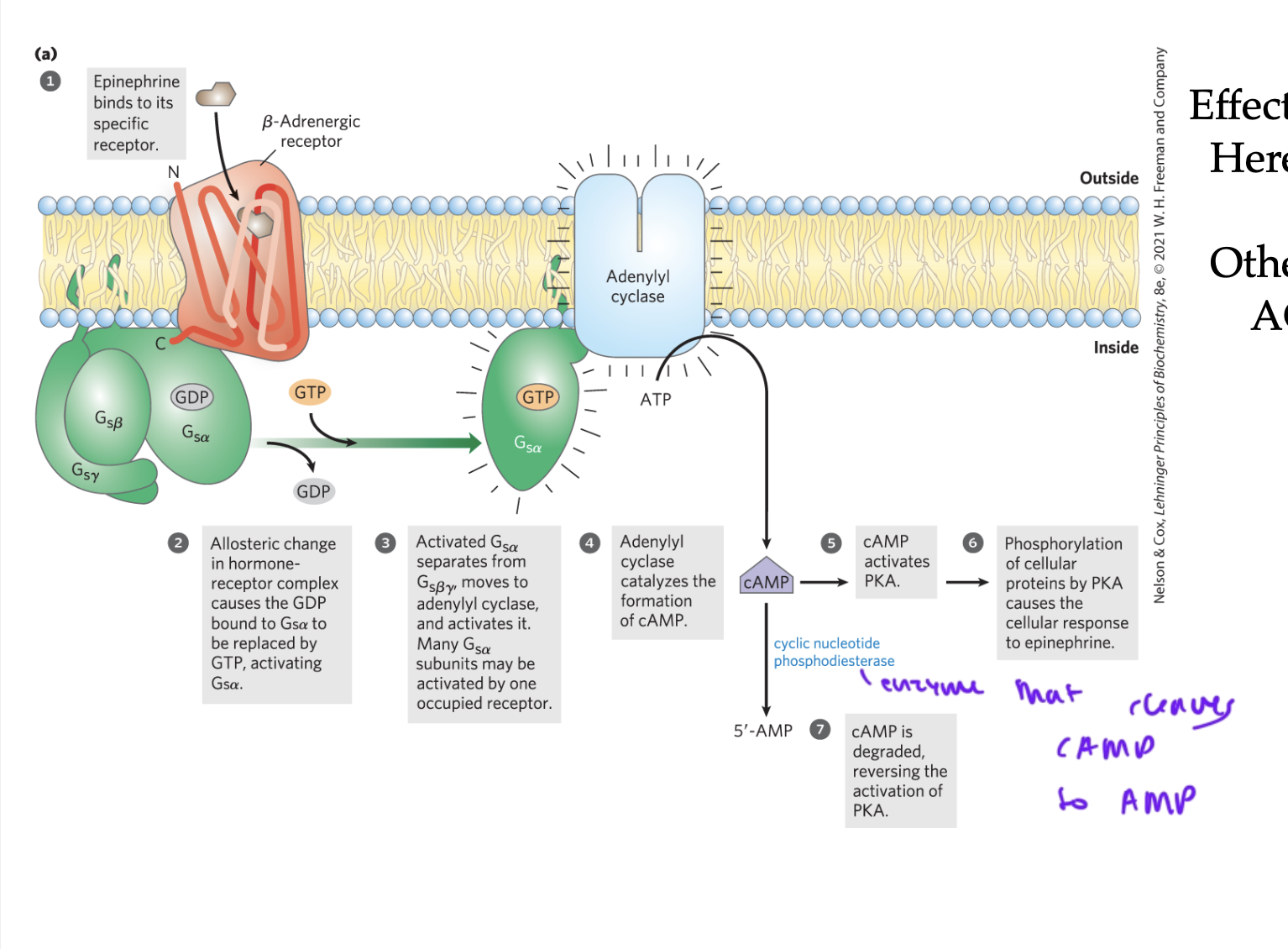
steps of GPCR activation
ligand (like epinephrine) binds to the beta-adrenergic receptor of the GPCR
the GPCR undergoes a shape change, and the GDP bound to the alpha subunit of the G-protein gets replaced with a GTP
alpha separates from beta gamma and activates adenylyl cyclase (the enzyme and effector)
adenylyl cyclase (AC) converts ATP to cAMP
cAMP activates PKA, which phosphorylates proteins causing a cellular response
cyclic nucleotide phosphodiesterase cleaves cAMP to form AMP so no more PKA gets activated
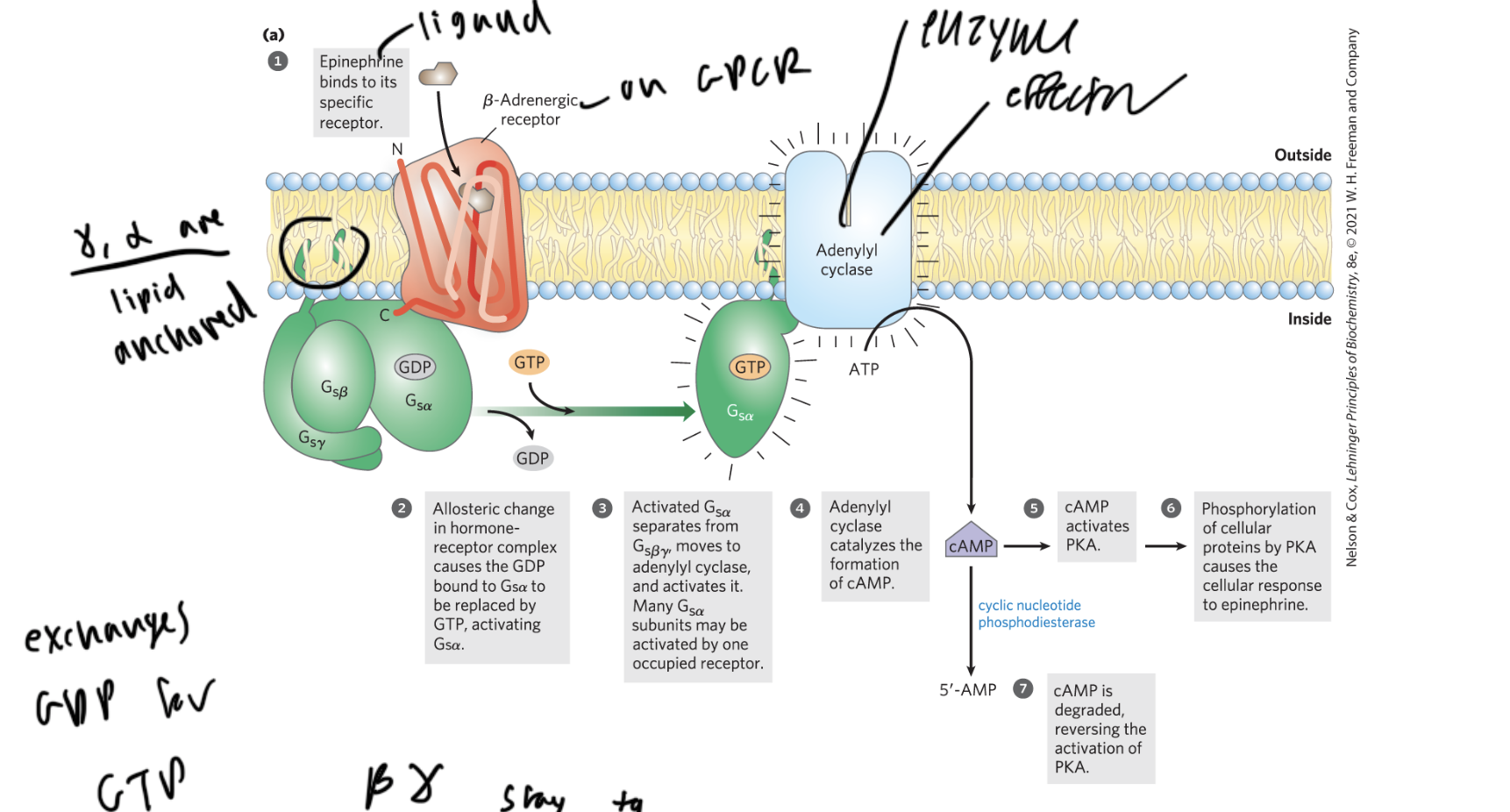
which two subunits of the g-protein in a GPCR are always together
beta and gamma
what causes the shape change of the alpha g-protein subunit
when GDP is exchanged for GTP
this is what causes the alpha to dissociate from beta gamma
Galpha subunit activity
active when GTP bound
the switches of the protein face in to keep the P inside
inactive when GDP bound
switches face out so the P can leave the protein to bind a new GTP
when the P leaves it can interact with beta gamma
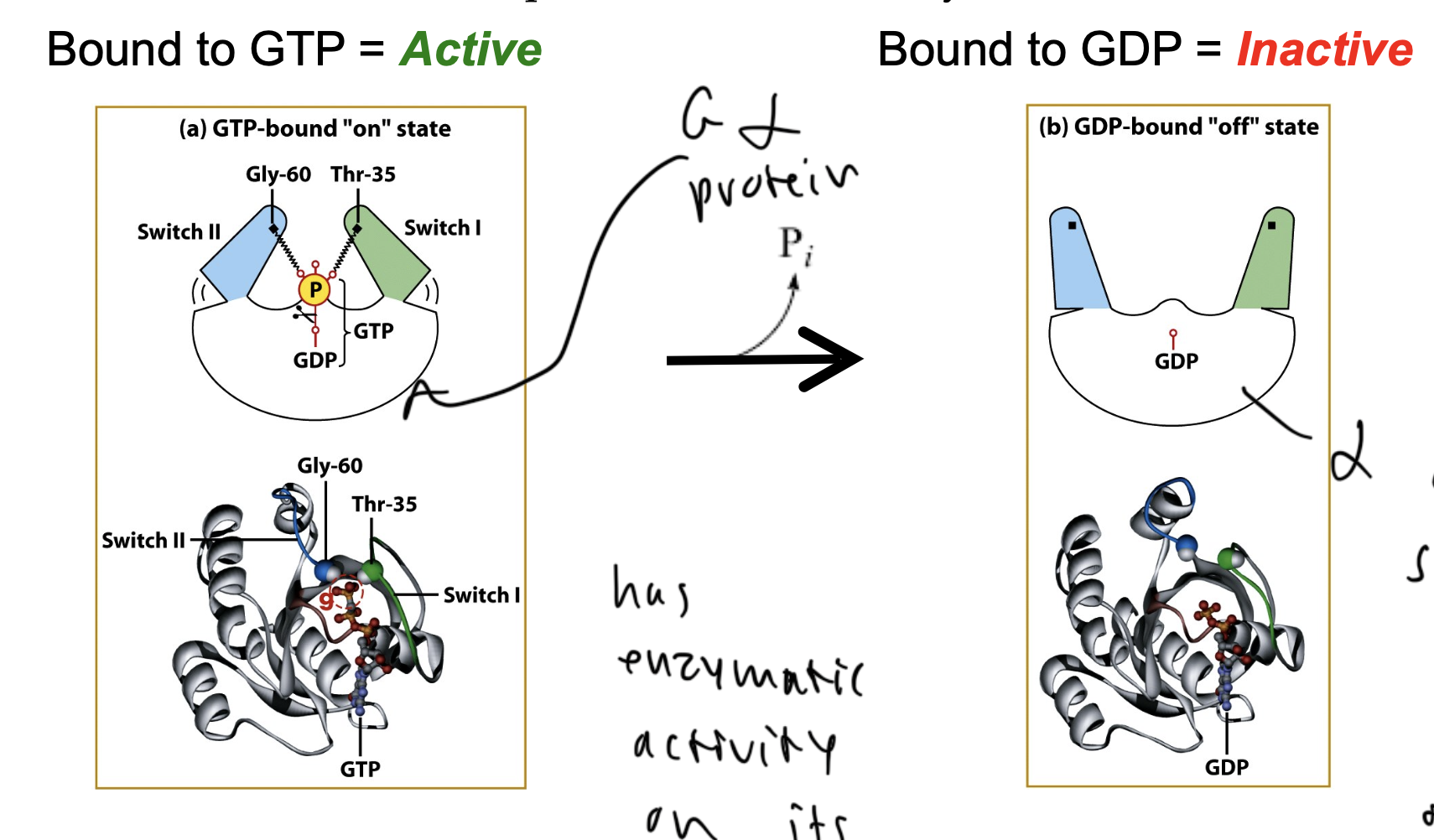
intrinsic rate of hydrolysis
when the last P leaves the Galpha protein after it binds a new GTP
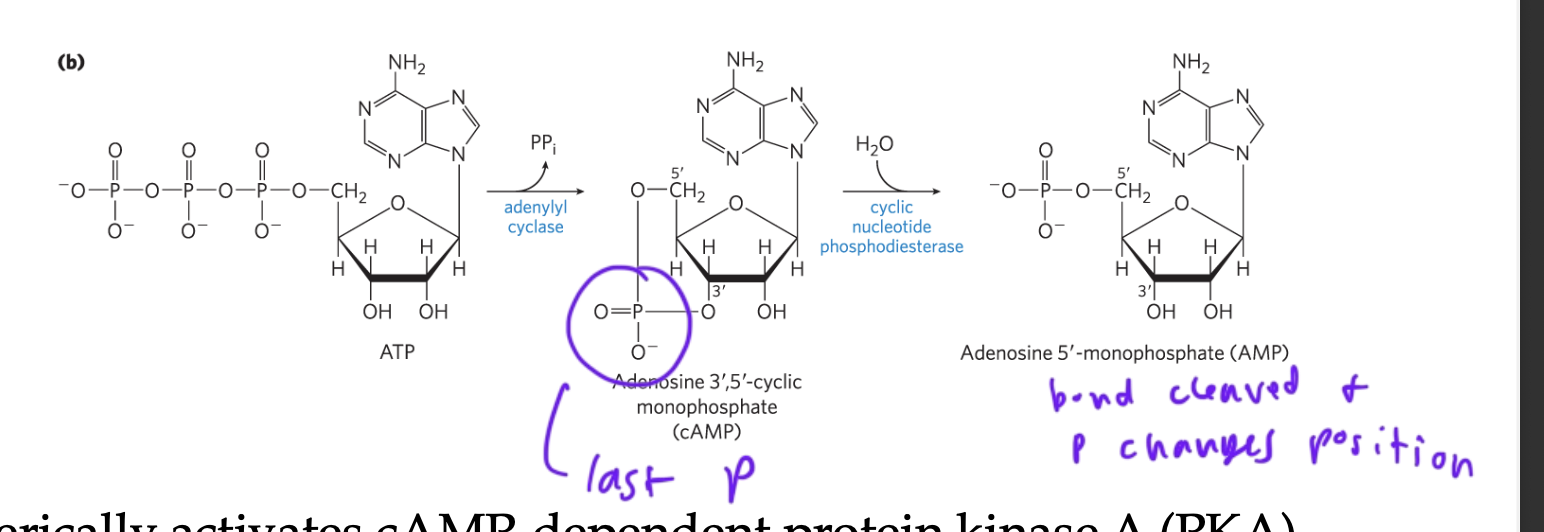
cyclic nucleotide phosphodiesterase
enzyme that cleaves cAMP to form AMP
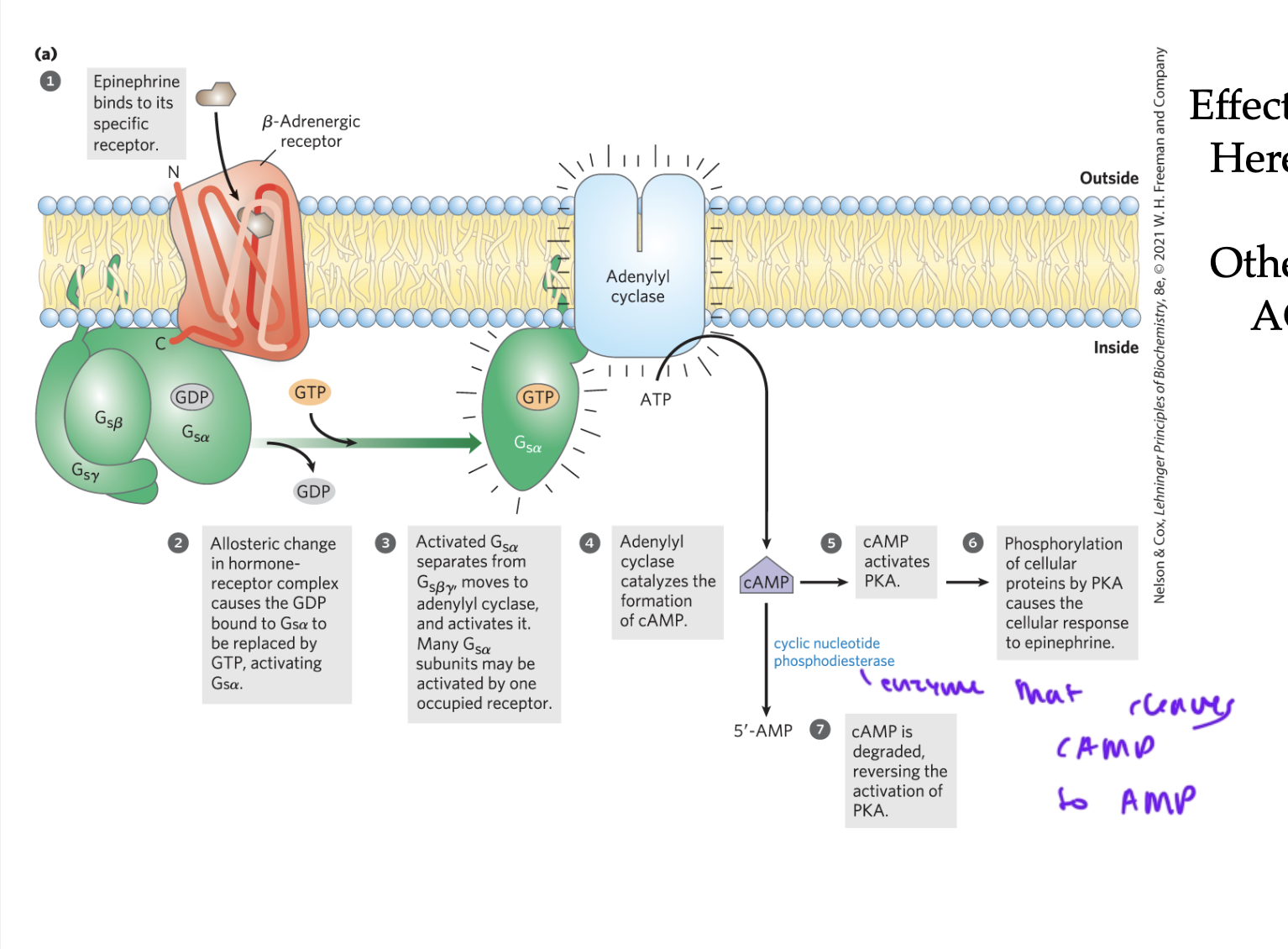
adenylyl cyclase (AC)
converts ATP to cAMP when activated by Galpha
cAMP is a common
secondary messenger
causes a quick response
many GPCRs mediate their effects via
cAMP (using Gs or Gi)
cAMP _____ activates PKA (cAMP-dependent protein kinase A)
allosterically
cAMP-dependent protein kinase A
PKA
when activated by/bound to cAMP, activates other enzymes that produce glucose from glycogen
cAMP being converted into AMP
adenosine-monophosphate
the location of the P changes since a bond it was attached to got cleaved
what does PKA need in order to be functional
have cAMP bound to it
protein kinases
add Ps to things, typically
Ser
Thr
Tyr
since these all have OH’s, which are easily phsophorylated
protein kinases differ in
the target proteins that they recognize
depends on the AA sequence of the target protein that the kinases recognizes
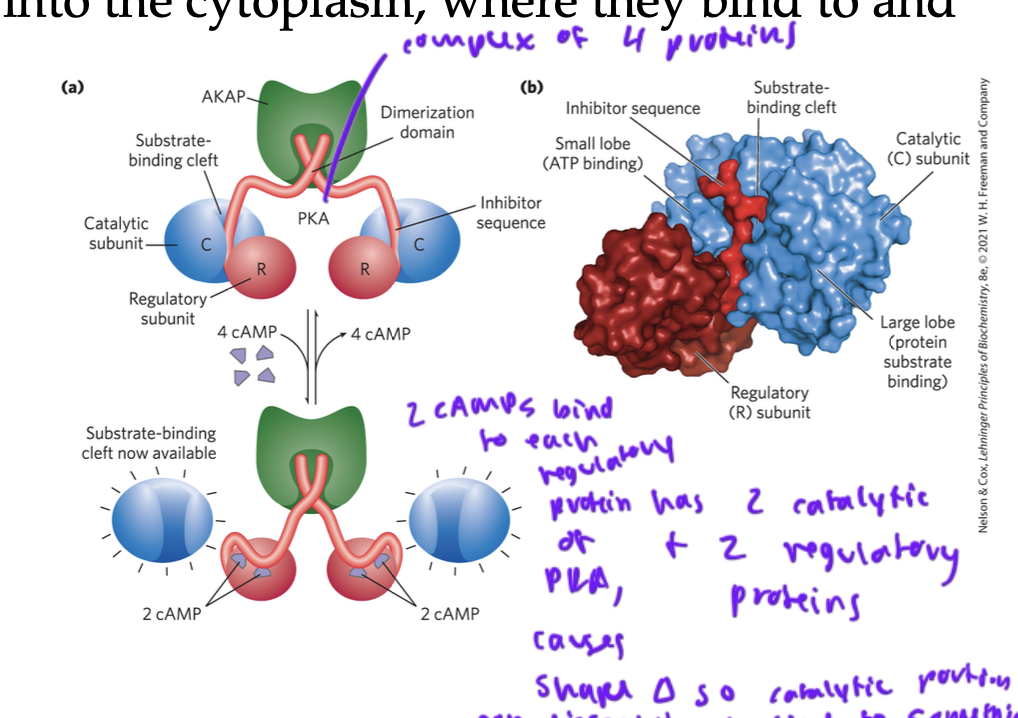
in the cAMP pathway, cAMP molecules diffuse into the _____
cytoplasm, where they bind to and activate PKAs
two cAMP bind to each regulatory protein of the PKA complex
once bound, this causes a shape change so that the two catalytic proteins of the complex can dissociate from the regulatory subunits and bind to something downstream (often activates proteins to trigger breaking down of glycogen in the liver for the sympathetic/ fight or flight response
PKAs can do many different things in
diff cell types
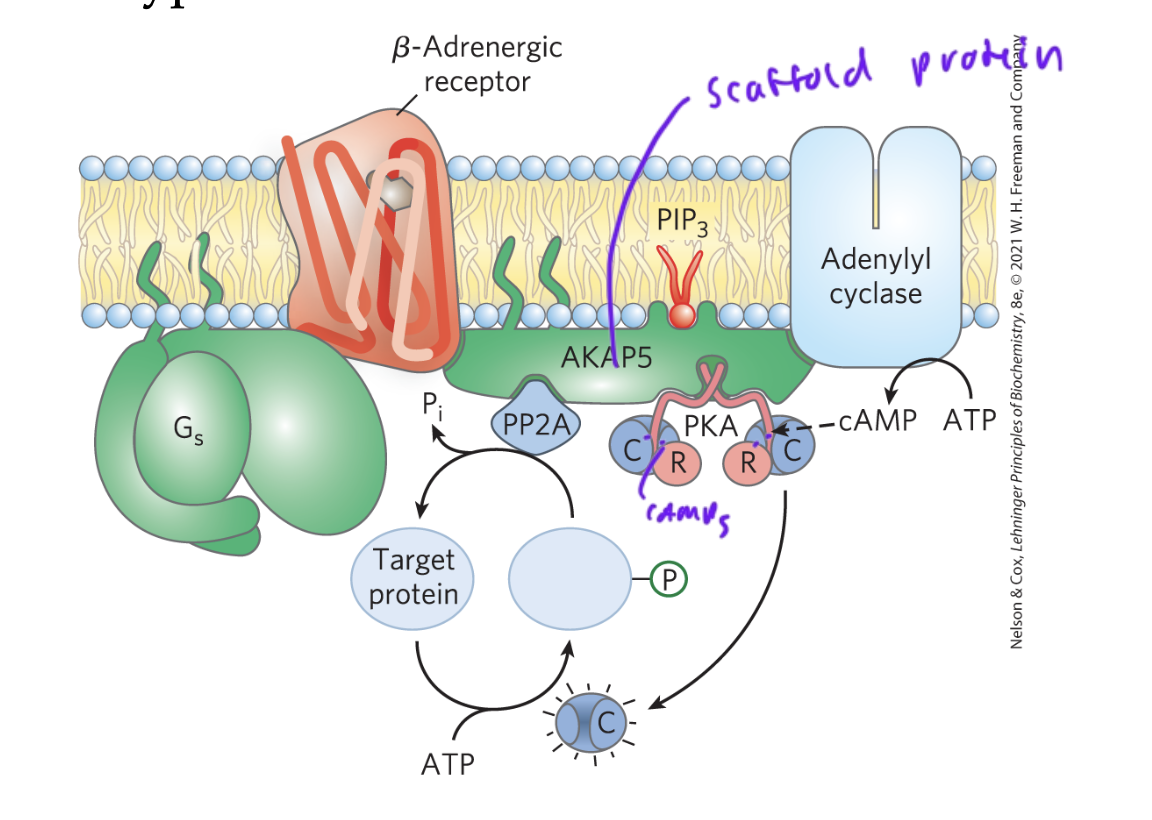
scaffold/anchoring proteins
cluster signaling proteins together to incr the rate of rxn
this localization of PKA via anchoring proteins, for example, allows cAMP to mediate multiple signals at once
diff anchors are expressed in diff cell types, so changes the downstream affect of cAMP in diff cell types
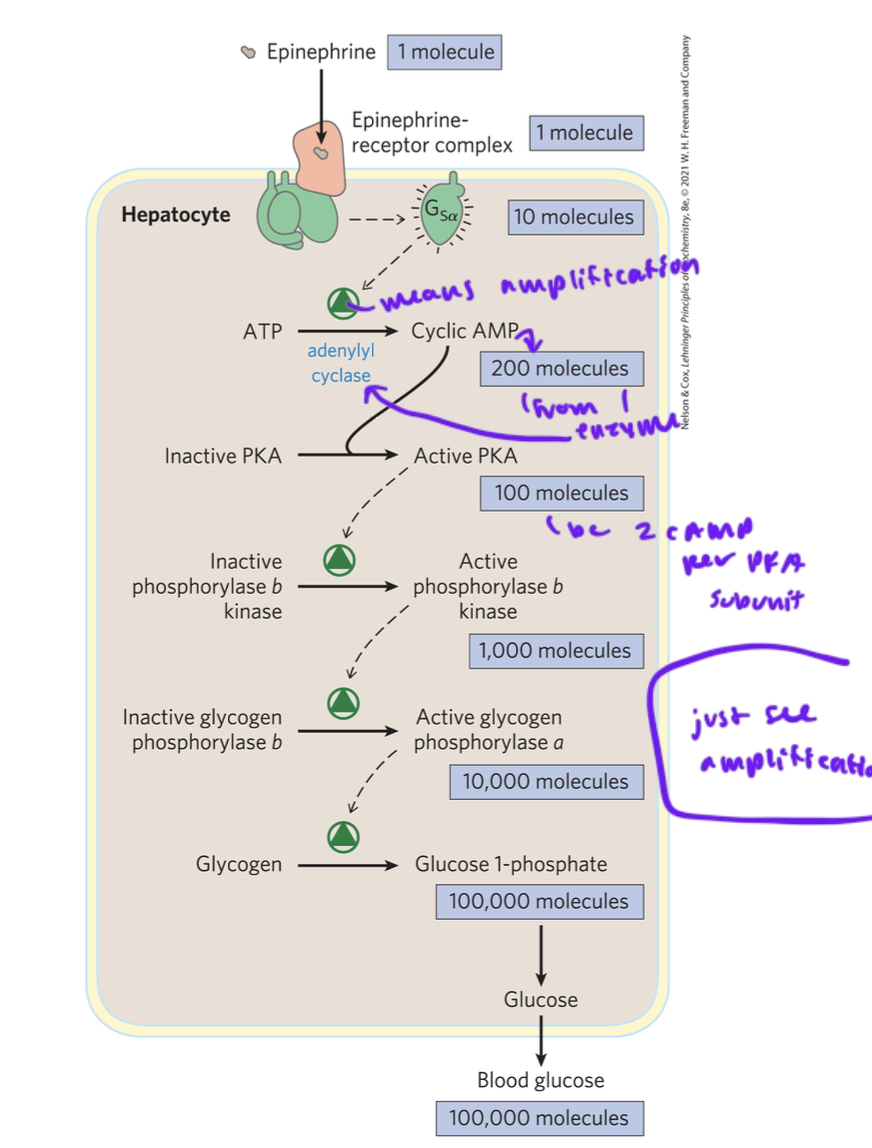
amplification in GPCRs
activation of just a few GPCRs can activate a few adenylyl cyclases which convert MANY ATP into cAMP
but it takes two cAMP to activate one catalytic subunit of PKA
then some PKA activates MANY kinases
then tens of thousands of glucose molecules can eventually get released into the bloodstream from the liver
diff cells can express diff ____, which bind the same ligand/hormone
GPCRs
hormone receptor-mediated responses regulated by G-proteins can be stimulatory or inhibitory since each hormone receptor specifically interacts with either a Gs or Gi protein
activation of Gi proteins can reduce signaling
basically GPCRs can use Gi or Gs proteins, therefore shutting down a pathway or stimulating one
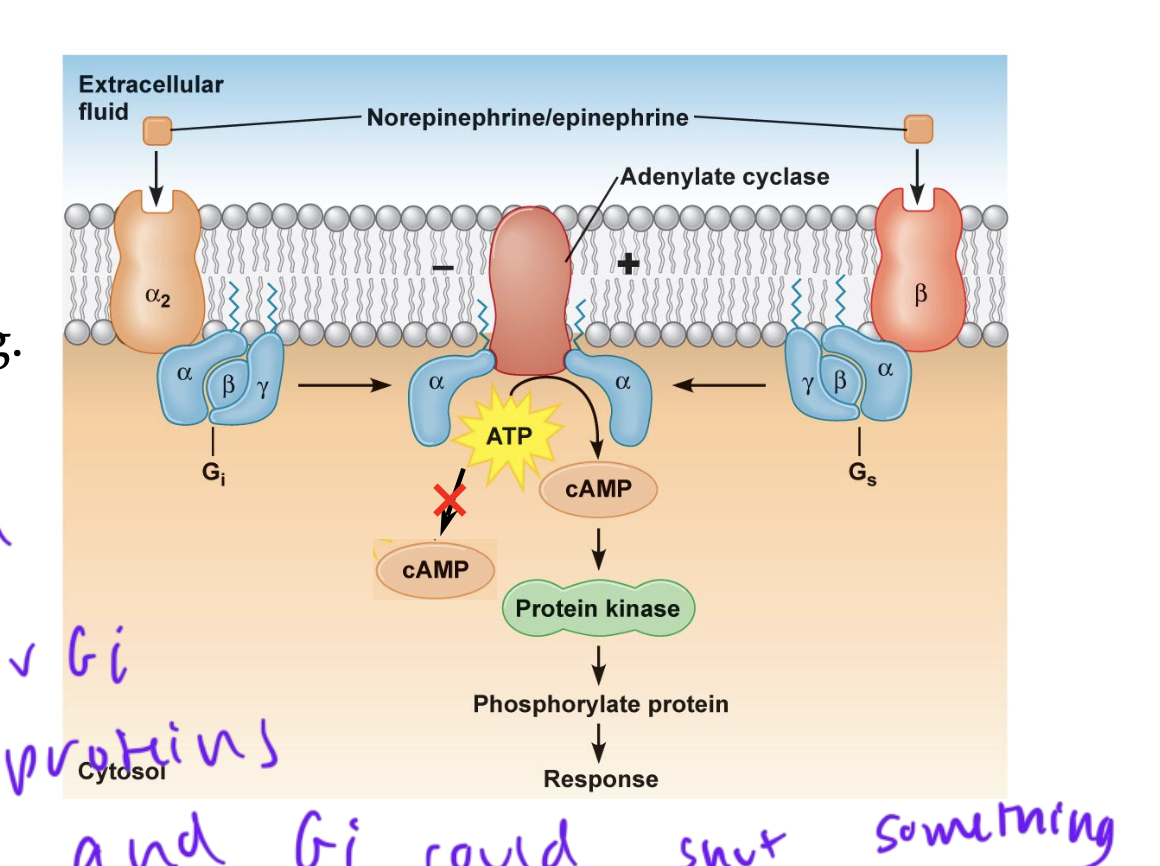
epinephrine is a ___ signal
short-acting
uses desensitization to turn off a pathway once it is no longer needed
ex: stopping glucose synthesis once the fight/flight response is no longer needed
turning off GPCR pathways via self-inactivation
pathway could be inactivated by lowering the [ligand]
but it is typically regulated by:
cyclic nucleotide phosphodiesterase converting cAMP into AMP, so there is less cAMP to activate PKAs
Galpha has intrinsic rate of hydrolysis, so it switches out its GTP for a GDP after a while (certain amount of time like a timer), and binding the GDP inactivates the pathway by down regulating cAMP levels since when Galpha binds GDP, it can no longer activate adenylyl cyclase to make cAMP
![<ul><li><p>pathway could be inactivated by lowering the [ligand]</p></li><li><p>but it is typically regulated by:</p><ul><li><p>cyclic nucleotide phosphodiesterase converting cAMP into AMP, so there is less cAMP to activate PKAs</p></li><li><p>Galpha has intrinsic rate of hydrolysis, so it switches out its GTP for a GDP after a while (certain amount of time like a timer), and binding the GDP inactivates the pathway by down regulating cAMP levels since when Galpha binds GDP, it can no longer activate adenylyl cyclase to make cAMP</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a99e9d7a-c895-4b73-825a-674e0f7db04c.png)
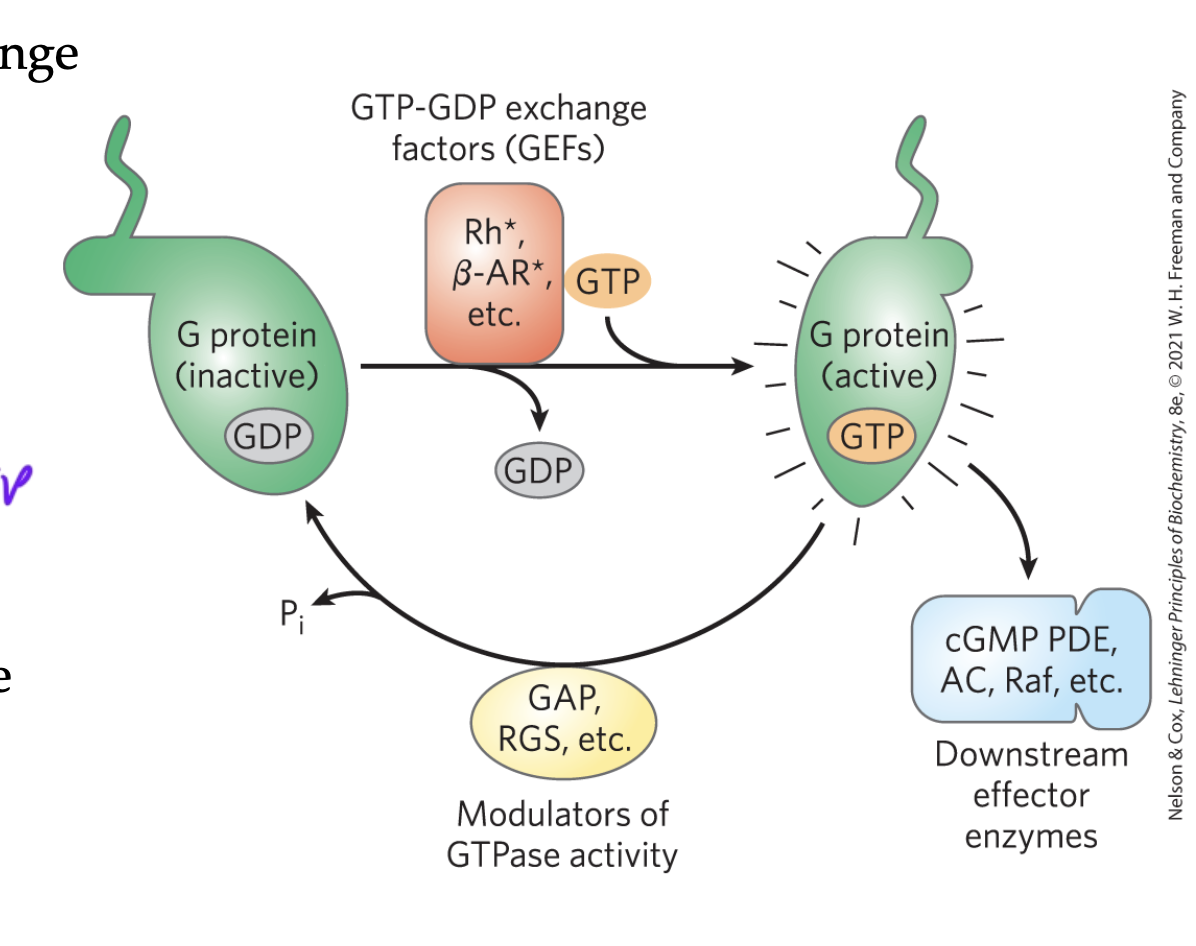
GEFs
guanine exchange factors
switch GDP for a GTP
usually activating but not always
ex: GPCR receptor binds ligand and then changes its shape so that alpha can bind GTP (the GPCR is the GEF)
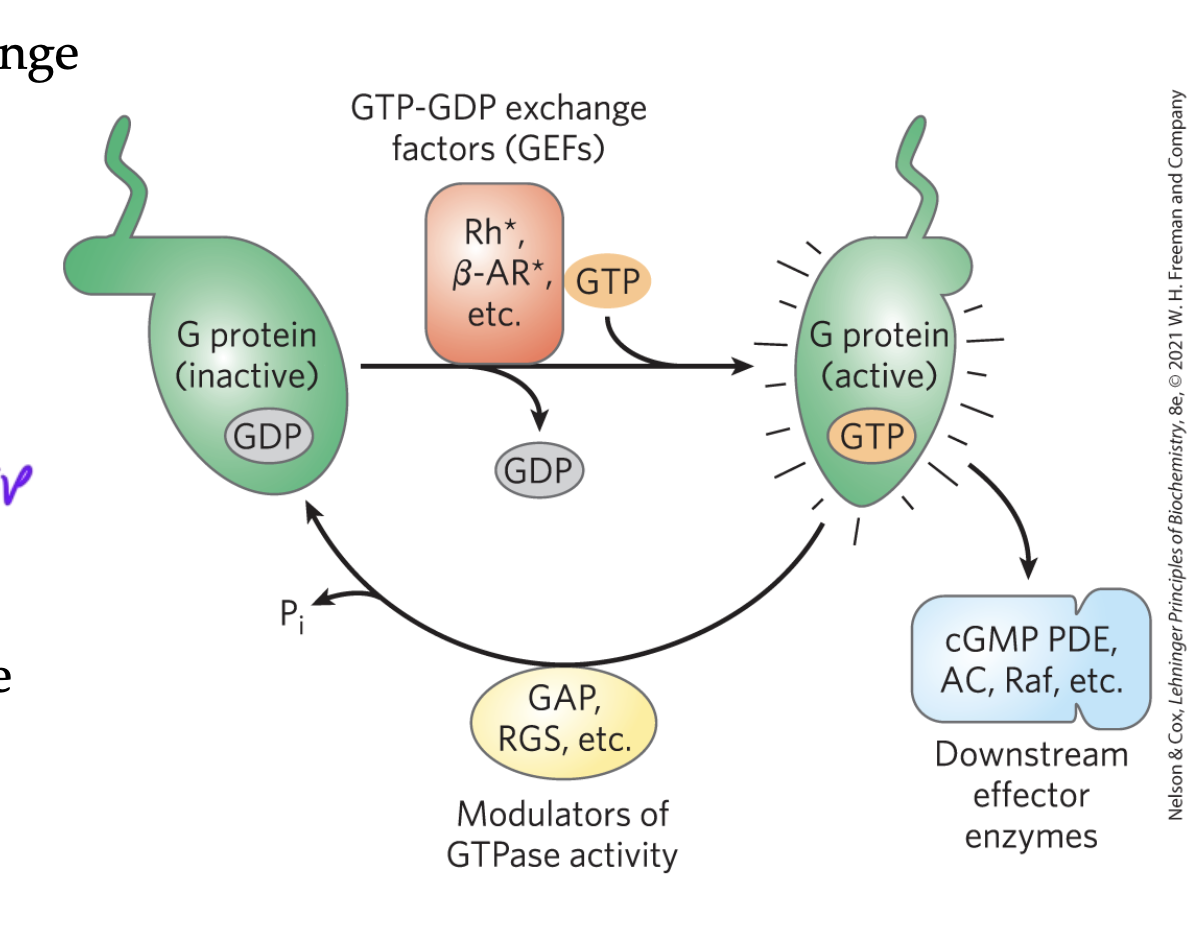
GAPs
GTPase activating proteins
switch GTPs for GDPs
usually inactivating
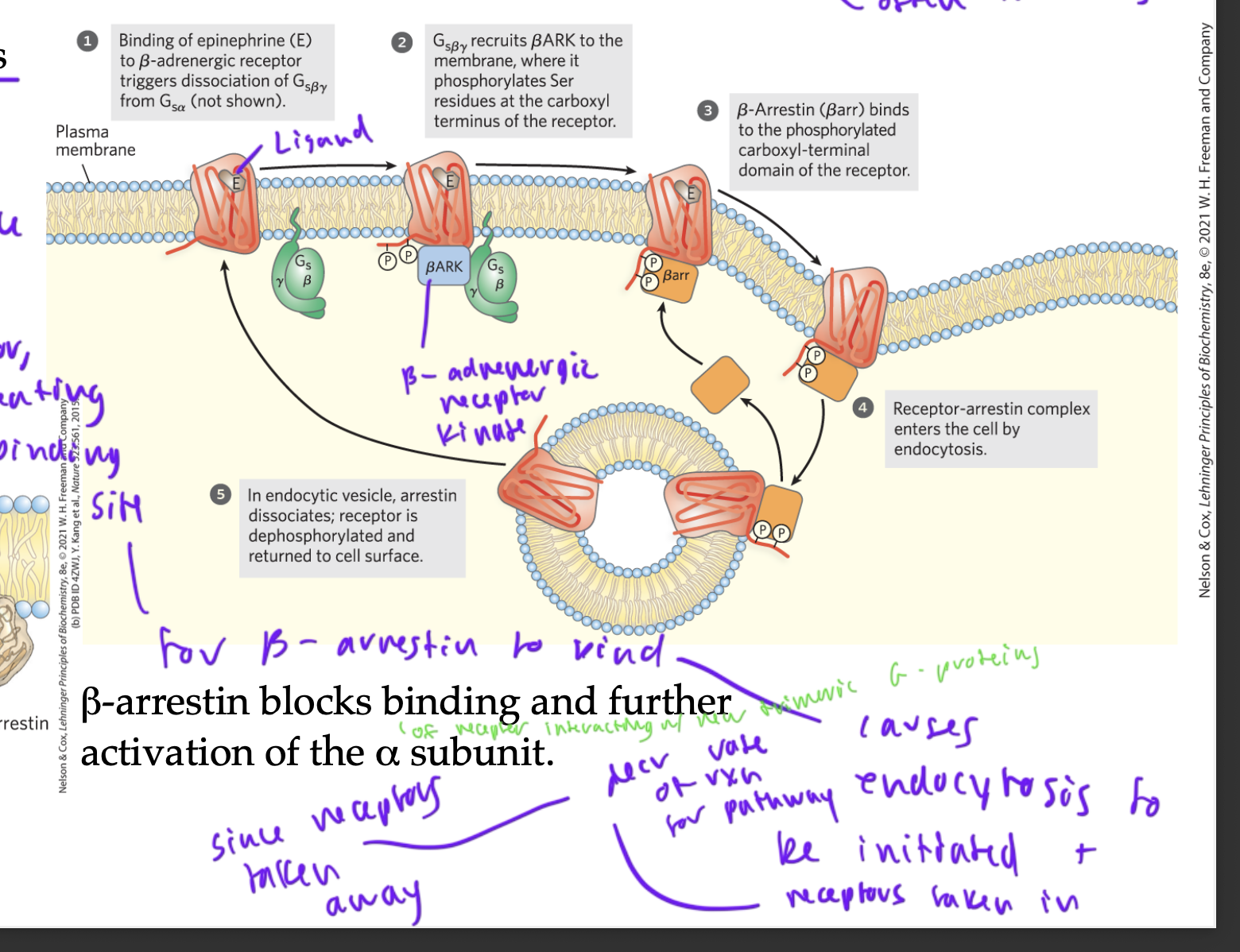
beta-adrenergic receptors to turn off the GPCR pathway (desensitization)
you can tell a pathway has been on bc beta and gamma G-proteins have been together for a while (so alpha must be bound to GTP and to adenylyl cyclase, keeping the pathway on) (also the ligand epinephrine is bound to the GPCR)
so BARK (beta-adrenergic receptor kinase) gets recruited and adds a P to the GPCR, creating a binding site
these binding sites allow beta-arrestin to bind, which initiates exocytosis at the membrane so that GPCRs are taken into the cell in a vesicle
this decr rate of rxn for the pathway since the receptors were taken away
agonists
structural analogs of ligands that bind to a GPCR receptor to keep the pathway going (mimics the ligand)
usually are drugs
antagonists
structural analogs of ligands that bind to a GPCR receptor so that the ligand cannot bind (the drug has very low Kd), and inhibits the pathway
often are drugs
morphine
an agonist of certain opioid receptors
therefore relieves pain since it causes feelings that combat the feeling of pain
opioid receptors in the body are often
GPCRs
GPCRs can bind to natural hormones such as endorphins and cause physiological affects like runner’s high, euphoria, respiratory depression
happens when mu-opioid receptor (a GPCR) gets signals and then causes downstream affects that cause good feelings (which combat the feelings of pain)
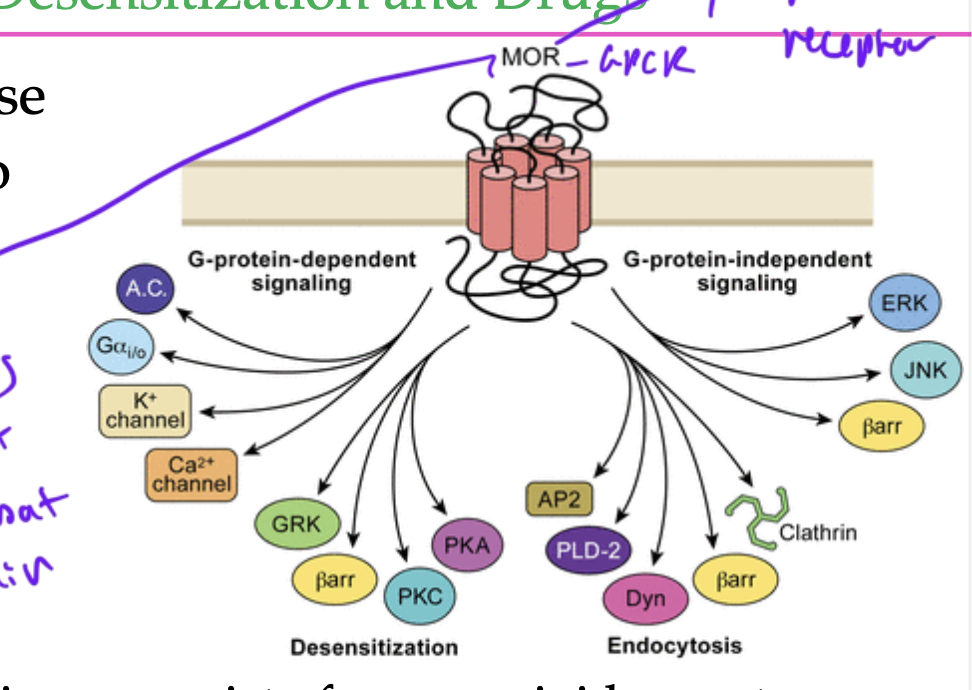
what is bad about GPCR opioid receptors?
the receptors can become desensitized if they are constantly receiving ligand/drug/hormone
this causes drug resistance and eventually addiction
second messengers
cAMP
IP3
DAG (diacylgylcerol)
hormone binds to a GPCR
alpha activated, interacts with an effector (phospholipase C) (PLC)
PLC’s substrate is pip2, a phospholipid
PLC cleaves polar head groups and phosphate of the glycerophospholipid (pip2)
the glycerophospholipid had an inositol head group, and once cleaved, it forms IP3
the remaing glycerol backbone with two FA chains forms DAG (diacylgylcerol)
DAG and IP3 are both secondary messengers
DAG stays in membrane due to its FAs, it diffuses out laterally through the membrane and interacts with protein kinase C (but DAG alone cannot activate protein kinase C)
IP3 binds to receptor-gated Ca2+ channel in ER membrane, causing gate to open and Ca2+ leaves ER
the Ca2+ also binds protein kinase C
now protein kinase C is fully activated
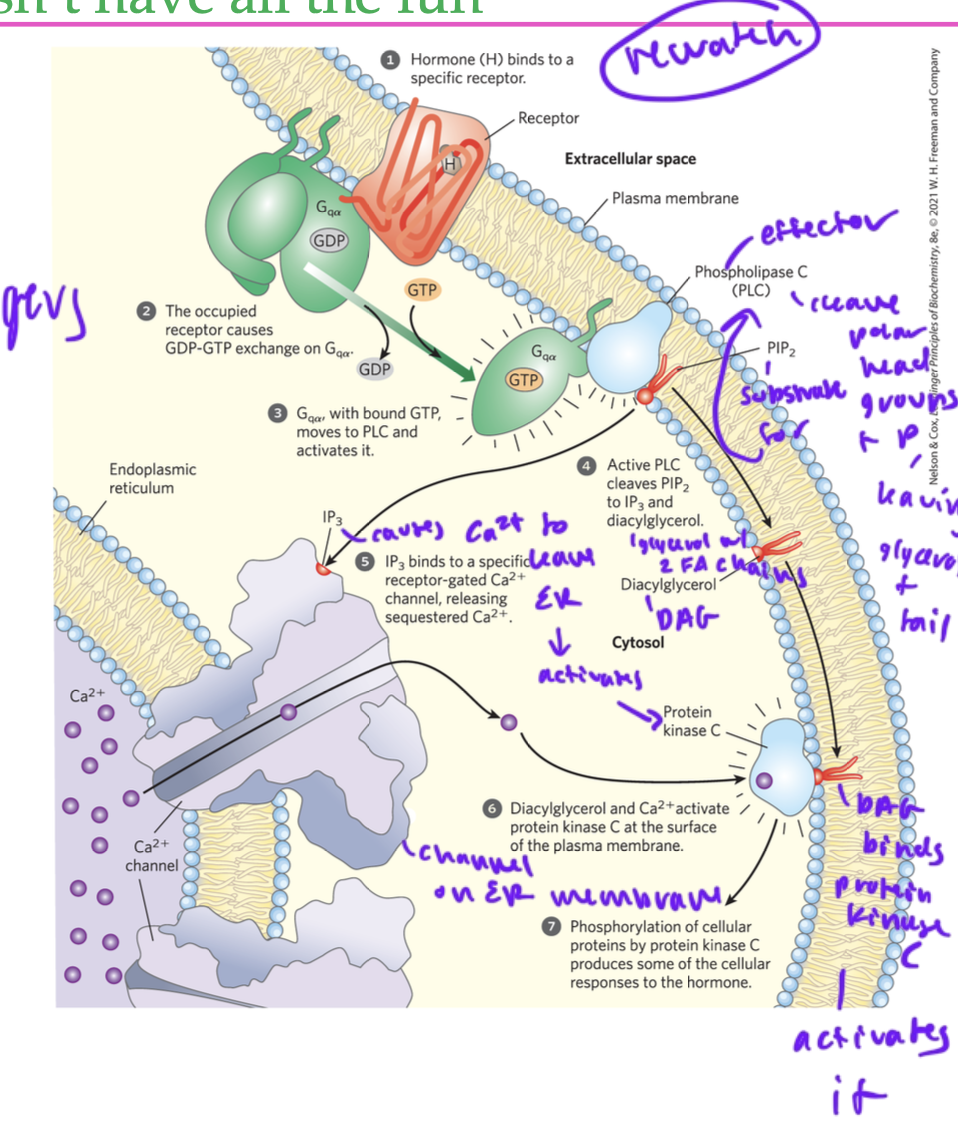
DAG and IP3 are ____ secondary messengers
lipid
so both are membrane permeable
activated effectors often generate
secondary messengers
aspects of one pathway can influence another
crosstalk
when are G-proteins activated?
when the GDP leaves and a new GTP binds to the Galpha protein
there are many categories of
enzyme-linked membrane receptors
can require one or two ligands
receptor tyrosine kinase activation
ligand binds to each of the two polypeptide receptors
the two receptors dimerize and cross phosphorylate each other at their tyrosine residues from ATP
the phosphates on the catalytic domains can serve as docking sites for downstream proteins
the receptors are formed or an extracellular ligand-binding domain and an intracellular catalytic domain
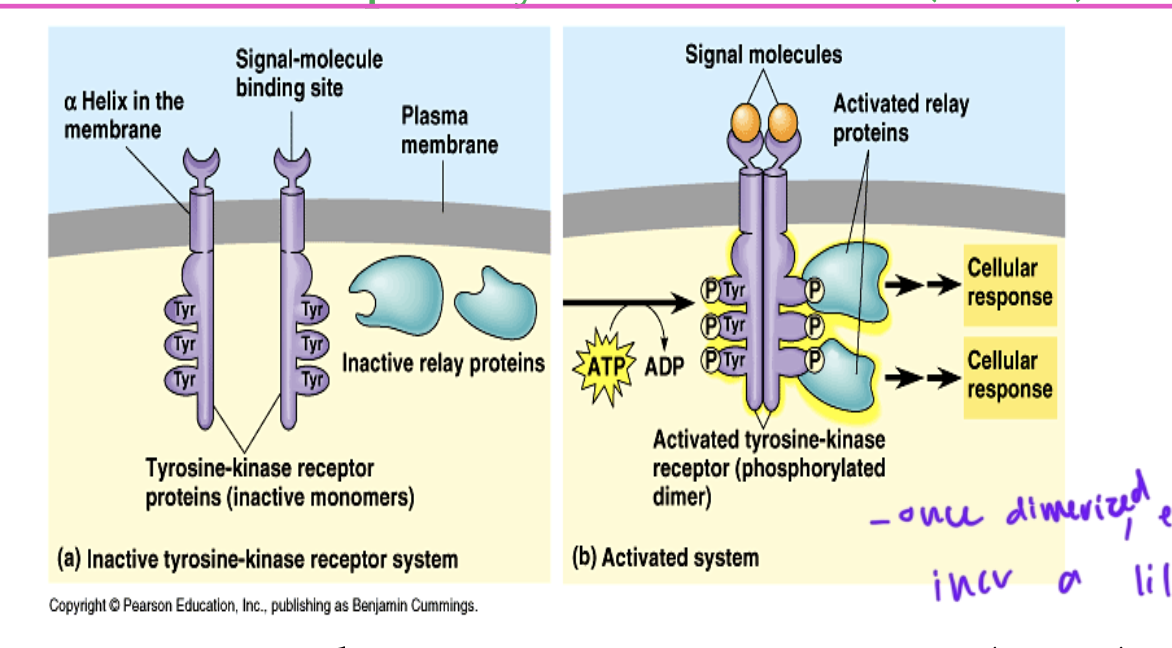
in tyrosine kinases, each receptor needs its own
ligand bound
once receptor tyrosine kinases (RTKs) are dimerized but not yet cross-phosphorylated, enzymatic activity…
incr a little bit so that they can cross-phosphorylate each other
so most RTK catalytic domains have low intrinsic kinase activity
one activated RTK dimer can activate
10 or more different intracellular proteins simultaneously
which can cause many diff responses
RTK pathways are especially helpful for
when a cell needs to regulate and coordinate a variety of pathways at once
IRS-1
a target protein for RTKs
insulin-receptor substrate-1
once the RTK gets phosphorylated, IRS-1 can bind to the P docking sites and can itself become a docking site for other proteins
the phosphorylated tyrosines of RTKs bind to ___ domains on target proteins
SH2
PTB (phospho-tyrosine binding) domain
these two types of domains are domains of target proteins that bind to the Ps of the RTKs
they have similar tertiary structure
SH2 and PTB can couple activated RTKs to downstream components of signal pathways
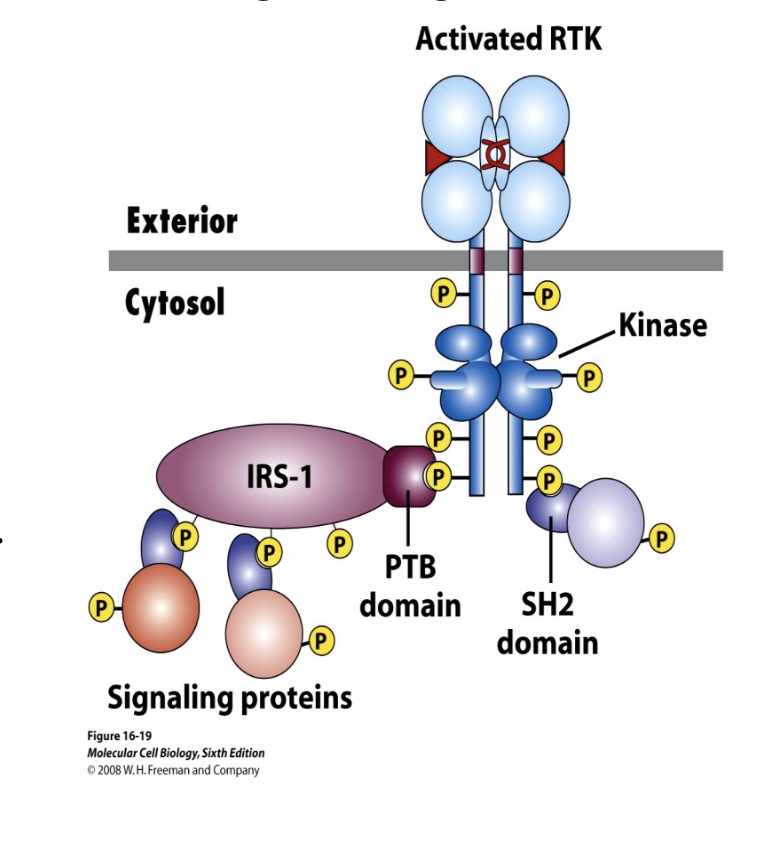
proteins that only bind to RTK when the RTK is phosphorylated have certain
domains that bind to the P
SH2 or PTB binding domains
phosphatases
remove phosphates
the opposite of kinases
examples of some second messengers
IP3
DAG
Ca2+
cAMP
phosphodiesterases
enzymes that break down second messengers
insulin is secreted in response to
high blood glucose
it is released from the pancreas and goes to liver, fat, and muscle cells by traveling through the bloodstream
diabetes
the inability to make or sense insulin
due to either a faulty receptor or an inability to produce insulin
binding of insulin to the insulin receptor causes
a cascade of events that lead to incr glu uptake and metabolism
RTKs are made up of _____ and ____ proteins
alpha and beta
one of each
but RTKs can vary in shape a lot
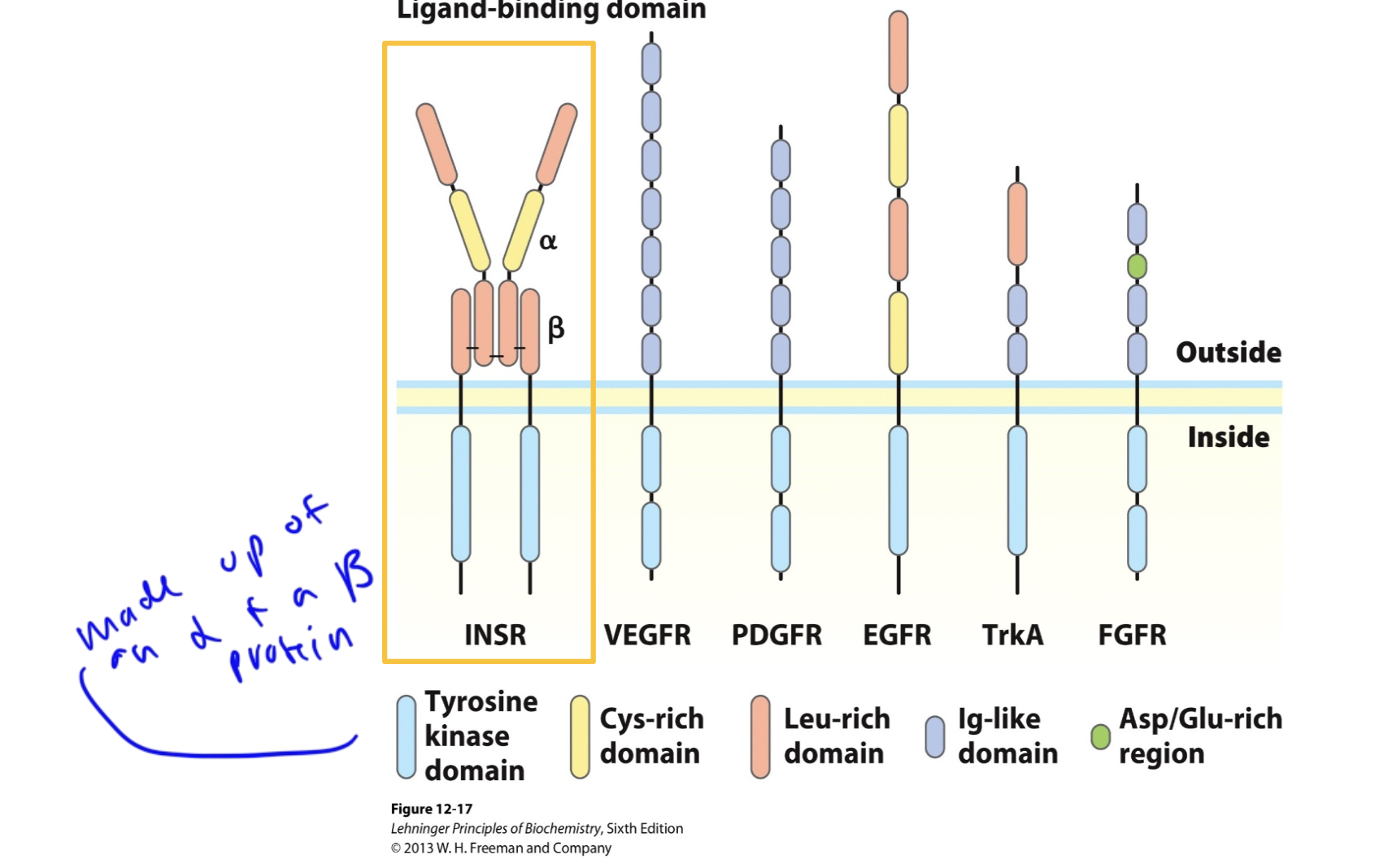
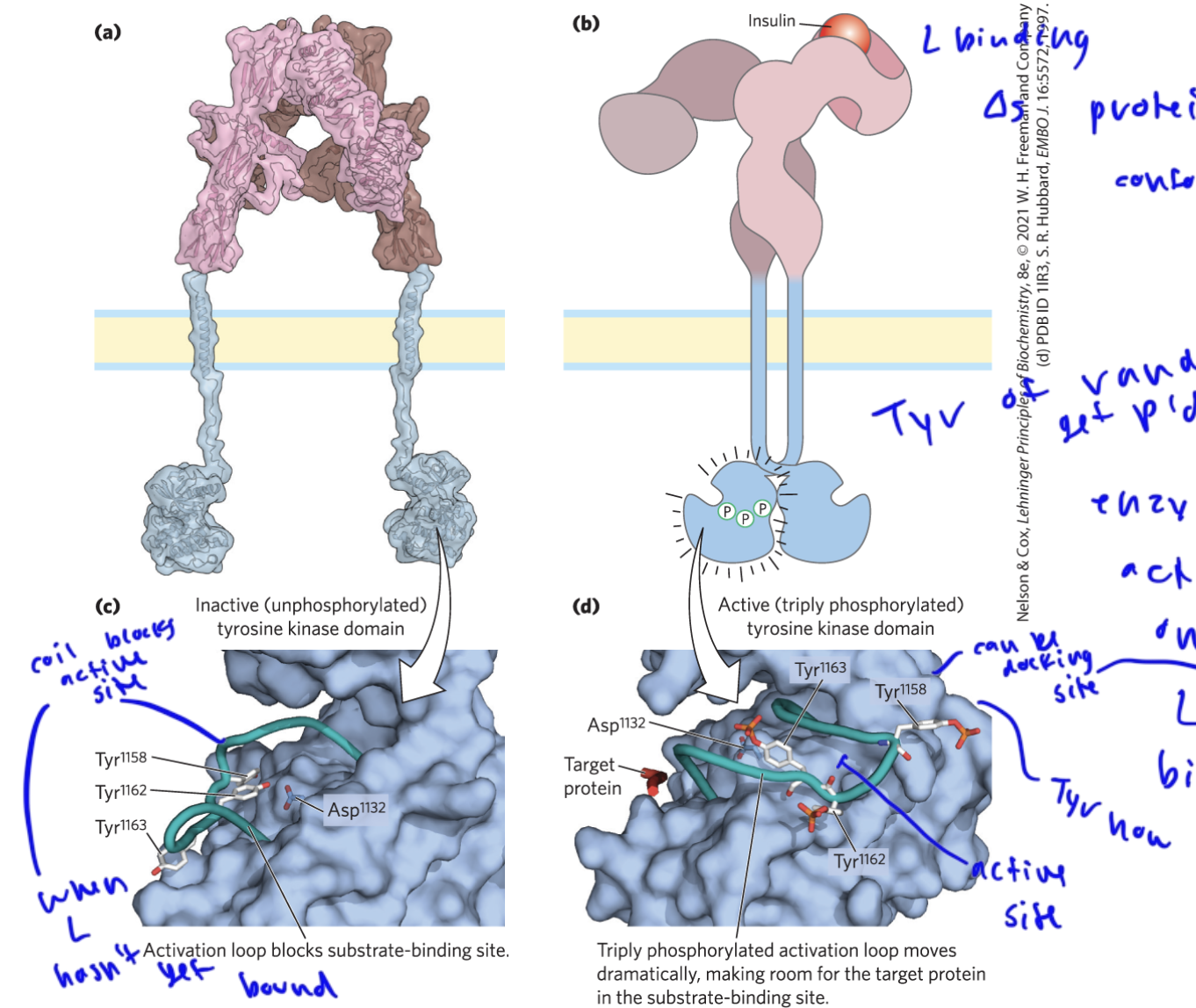
insulin receptor structure
is an RTK and the ligand binds to one of the extracellular proteins of the receptor
once L binds, the two intracellular proteins move closer to each other and then phosphorylate each other
this phosphorylation at the tyrosines on the protein intracellular random coil causes the active site of the intracellular domains to open up, as the Tyr are no longer covering it
before the random coil with the Tyr’s was covering the active site
the P’d Tyr’s can serve as docking sites for SH2 and PTB binding domains
Insulin pathway to signal for GLUT 4 transporter production
insulin binds to the insulin receptor, which autophosphorylates itself at its Tyr residues
the receptor then Ps the IRS1 on its Tyr residues, so the IRS1 basically docks into the Tyrs
The SH2 domain of Grb2 (scaffold protein) binds to the P’d Tyr of IRS1 (so Brb2 brings IRS1 and Sos tg)
Sos binds to Grb2 and acts as a GEF to switch out Ras’s GDP for a GTP
so Sos activates Ras
activated Ras binds to Raf-1, which acts as a kinase to phosphorylate MEK on its two Ser residues
MEK is also a kinase and Ps ERK at its Thr and Tyr
ERK goes into the nucleus and Ps and activates a TF, Elk1, which can now associate with another TF, SRF and cause expression of GLUT4 transporter genes
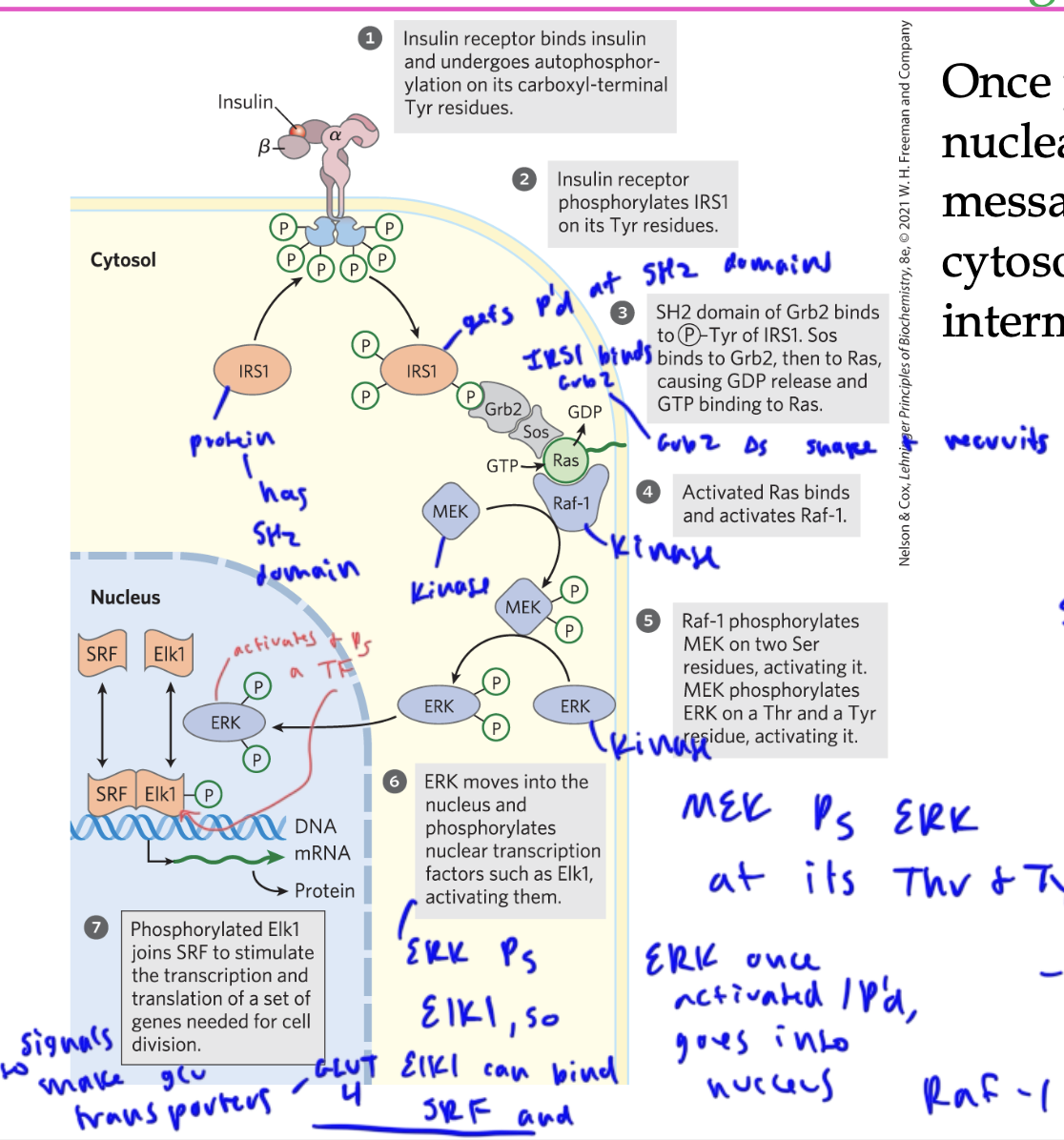
____ is the point of nucleation for many other proteins in the insulin pathway
IRS1
Grb is a ____ protein
scaffold/ adaptor
it also has a SH2 domain, allowing it to bind to IRS1
Grb2 brings IRS1 and Sos tg