BMS1052 - W2: Communicating with Action Potentials
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Explain some properties of neurons (in terms of conduction and action potentials).
substrate: intracellular fluid/ cytosol
charge is carried by ions (Na+, K+, Ca2+, Cl-)
Poor insulation between intra- and extracellular space (charge flow along neurite and across membrane matters)
Properties:
thicker axons have lower resistance to charge movement and therefore will conduct faster
Temperature affects conduction. Warmer axons conduct faster.
What are the intracellular and extracellular spaces of neurons separated by?
A membrane - phospholipid bilayer
hydrophilic heads (phosphate)
hydrophobic tails (carbon chains)
Ion channels
What are they made up of?
How do they allow charged molecules to pass through?
amino acids (linked by peptide bonds) produce a polypeptide chain. Multiple polypeptide chains can associate to form subunits. Subunits can come together to form ion channels.
Ions can only easily cross the phospholipid membrane via specialized proteins that form ion channels an pumps.
Due to the hydrophilic and hydrophobic nature of the membrane, both ions and water will be unable to cross the membrane.
membrane-bound channels act as gatekeepers.
How can membranes be selectively permeable?
E.g. Sodium channels have a quite narrow internal structure which partly prevents movement through it. But there are also effective sodium binding sites that only allow positively charged ions to move through. And it has to shed the associated water molecules before it will actually physically fit through. So it is selectively permeable for a specific charge and size of ion.
What are aquaporins?
Water cannot directly cross the membrane, but can easily cross the membrane through specialized channels called aquaporins.
allow for the free flow of water across the membrane.
blocked aquaporin can lead to diseases
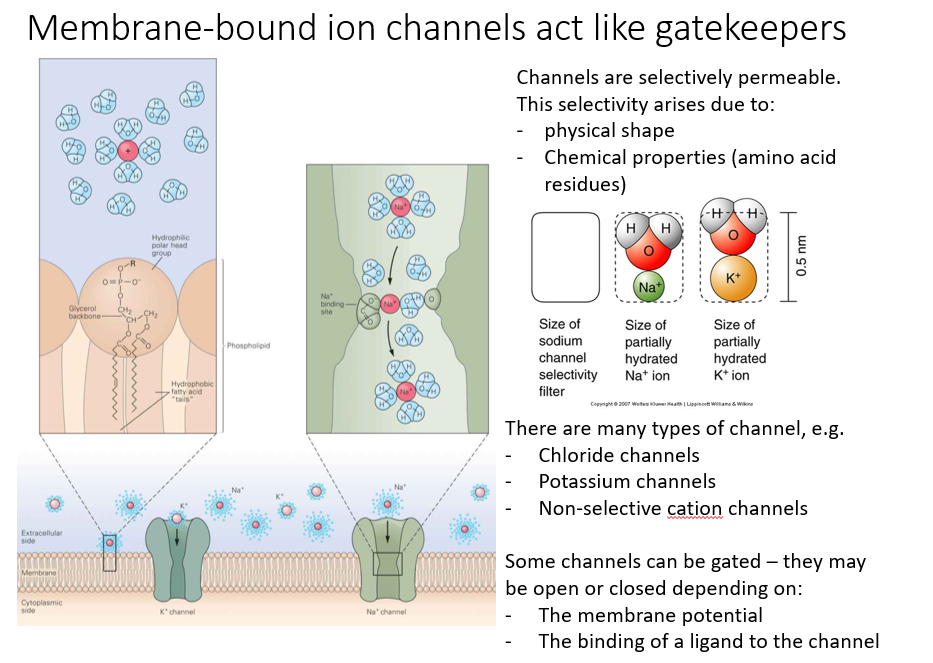
Assuming the membrane has ion channels, what will happen?
The ions will move down the concentration gradient (from left to right) until equilibrium is reached.
At equilibrium, there is still a movement of ions, but no net movement of ions. Se ions are still crossing the membrane, will be balanced.
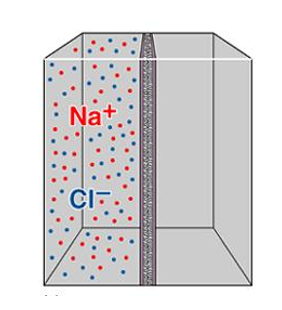
Aside from concentration gradients, what other factor can affect ion movement?
Electrical field across membrane.
charged ions will move to their respective anode and cathode.
if this is done in a compartment with ion channels, then we will be setting up a concentration gradient.
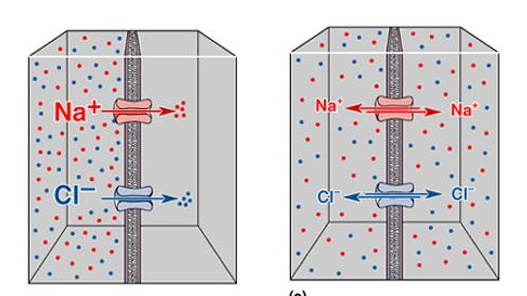
Charge and concentration gradient can affect ion movement, but how do these factors balance out at equilibrium?
Explain using image what happens if a sodium channel is added.
If a sodium channel is open, we would expect sodium to move down concentration gradient form right to left. But that is going to set up a charge imbalance (slightly higher positive charge on left). This is going to counteract further movement of sodium ions from right to left.
So at equilibrium, the change imbalance associated with just the movement of sodium ions balances out the concentration gradient.
Keep in mind this imbalance occurs because we’ve only opened up a sodium channel, and the generic anions are not moving across the membrane .
Nernst equation:
This can be used to predict the membrane potential at equilibrium (This relates to the previous flashcard)
this is assuming that the membrane is permeable to a single ion (which is rarely the case)
[IMPORTANT]: The Nernst equation is a ‘theoretical’ potential. The actual observed membrane potential will depend on multiple ions and all those ion’s permeability.
What do we need to know to use the Nernst equation?
ionic concentrations (inside and outside the cell)
ionic charge of ion of interest
Nernst equation in image
Eion is the voltage that counteracts movement of an ion due to its concentration gradient (and is used to refer to the ionic equilibrium potential for a particular ion)
so if a membrane is permeable to only one ion, then the membrane potential will move towards that ion’s equilibrium potential.
And if the membrane potential equals the ion equilibrium potential, there will be no net movement of that ion.
![<p>This can be used to predict the membrane potential at equilibrium (This relates to the previous flashcard)</p><ul><li><p>this is assuming that the membrane is permeable to a single ion (which is rarely the case)</p></li><li><p><strong>[IMPORTANT]:</strong> The Nernst equation is a<strong> ‘theoretical’ potential.</strong> The actual observed membrane potential will depend on multiple ions and all those ion’s permeability.</p></li></ul><div data-type="horizontalRule"><hr></div><p>What do we need to know to use the Nernst equation? </p><ol><li><p>ionic concentrations (inside and outside the cell)</p></li><li><p>ionic charge of ion of interest</p></li></ol><p></p><p>Nernst equation in image</p><div data-type="horizontalRule"><hr></div><p>E<sub>ion</sub><span> is the voltage that counteracts movement of an ion due to its concentration gradient (and is used to refer to the ionic equilibrium potential for a particular ion)</span></p><ul><li><p>so if a membrane is permeable to only one ion, then the membrane potential will move towards that ion’s equilibrium potential.</p></li><li><p>And if the membrane potential equals the ion equilibrium potential, there will be no net movement of that ion.</p></li></ul><p></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1156760a-a83b-4d22-854b-b2d3366ffd21.png)
What are the 4 most important ions in terms of neuronal signalling?
K+
Na+
Ca2+
Cl-
Do we measure the membrane potential of the cell from the inside or outside?
By convention, we measure the membrane potential of a cell from inside relative to the outside.
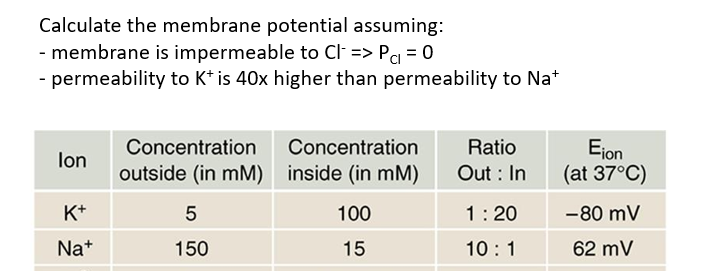
The Goldman-Hodgkin-Katz (GHK) equation.
We can calculate the membrane potential using the Goldman equation
What do we need to know to use the Nernst equation?
ionic concentrations (inside and outside the cell)
The relative membrane permeability for each ion
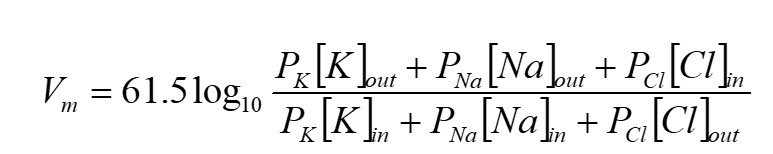
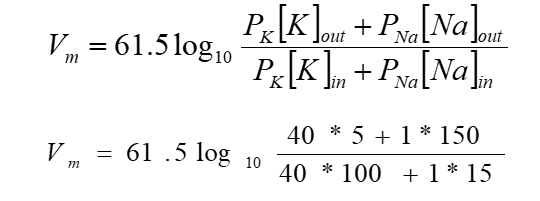
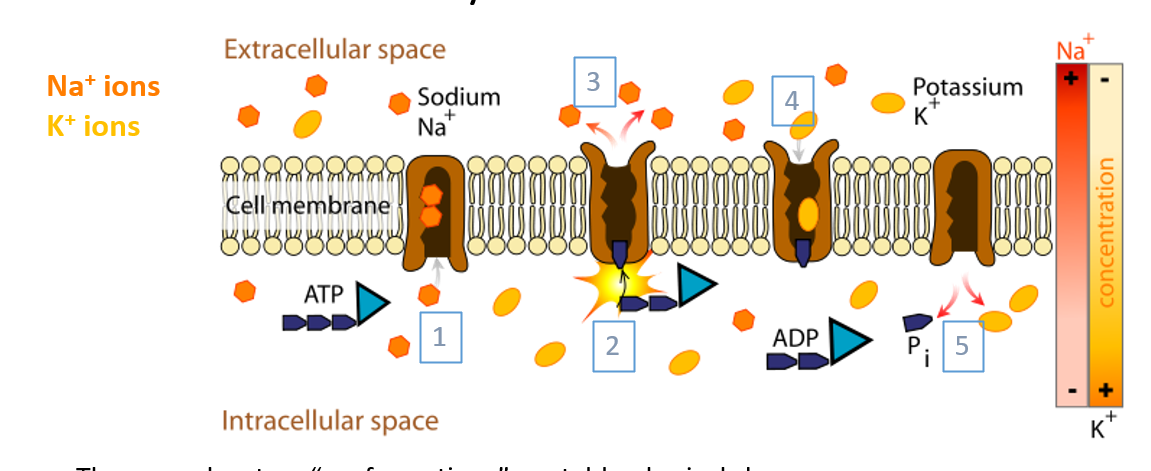
Na/K-ATPase
A pump that maintains the resting membrane potential and concentration gradient.
powered by ATP
pushes Na+ out of cell and K+ into cell
Has 2 conformations/ states:
facing inwards
facing outwards
IMAGE:
When facing intracellularly, pump binds ATP and 3 intracellular Na+ ions
ATP hydrolyzed, leading to phosphorylation of pump and release ADP
Once bound, it will change conformation and release Na+ to extracellular space
The pump binds 2 extracellular K+ ions, causing dephosphorylation and a second conformational change
ATP binds and K+ released to intracellular space
Potassium channels:
All K channels have 4 subunits with pore loops that regulate permeability
Types of K channels:
Two-pore-domain potassium channels
-contains 2 pore loop domains, which are generally open
-contribute to the ongoing K+ leak
-15 known types of these channels
Voltage gated potassium channels
-open state depends on membrane potential
-normally closed at resting membrane potential
-also called ‘delayed-rectified’ channels (because they take some time to open and return membrane potential to resting level)
calcium-activated potassium channels
-ligand activated (the ligand here is calcium)
-presence of calcium triggers channel opening
Inward rectifying potassium channel
-passes positive charge more easily into than out of the cell
Voltage gated sodium channels
opens when the membrane potential depolarizes
Has 3 states: Open, closed or inactive
has a pore loop (red in image) which acts as a selectivity filter (only allows sodium ions to pass through)
What are the parts of an action potential?
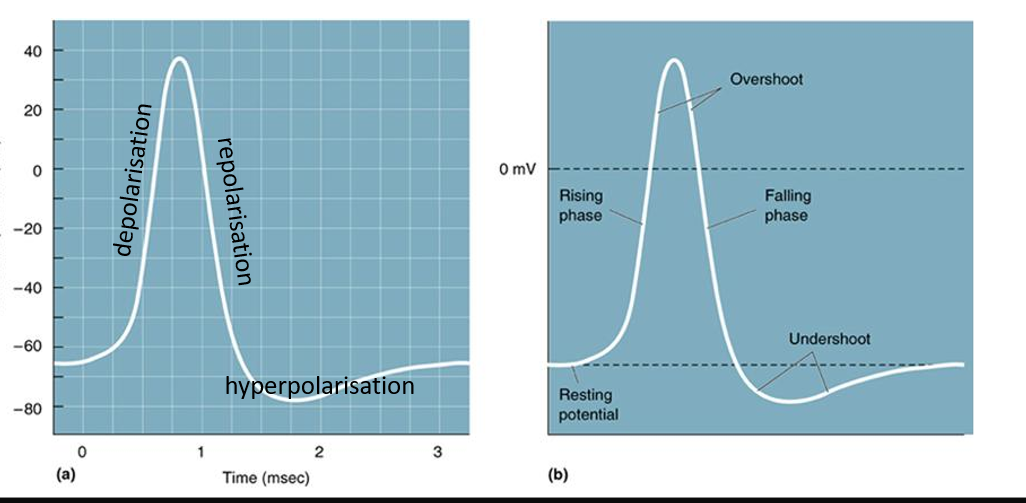
How do we get signals from point A to point B along a neuron (from cell body to axon terminal)
Option 1: Passive
diffusion of ions through the cytosol
requires no energy
has problems (slow, breaks down over long distance because of leak membrane, unreliable)
Option 2: action potentials
propagates, reliable but requires energy
not instantaneous, but pattern and timing of the action potentials is preserved as they transfer along the axon.
Describe an action potential - the steps and channels involved.
depolarization to threshold
-Action potential begins when the membrane is depolarized past a threshold that triggers opening of voltage gated sodium channels.
-Action potentials either happen or they don’t (if threshold is crossed, then action potential occurs)
-Sodium channels opening → membrane is more permeable to sodium then potassium → membrane potential rapidly approaches Na equilibrium potential (ENa).
How does this happen? What causes initial depolarization?
option 1: physically gated sodium channels open (e.g. stretch sensitive sodium channels in the skin)
option 2: depolarization may be inherited from elsewhere in the neuron
Rapid depolarization (voltage gated sodium channels open)
-when the Na channels open P(Na) » P(K)
-as membrane continues to depolarize (as Na rushes into the intracellular space), more voltage gated Na channels open
Repolarization (Voltage gated sodium channels close)
-voltage gated sodium channels only open for ~1ms, then they close and inactivate (globular protein blocks the pore)
-So voltage of membrane does not reach the equilibrium potential of sodium.
-instead, as the Na channels close, the membrane becomes more permeable to potassium. So the membrane potential heads back down towards the equilibrium potential of potassium.
Hyperpolarization (Kv delayed rectifier K channels open)
-while the delayed rectifier K channels are triggered to open quite early (~-45mv), their opening is delayed.
-When they finally open, the membrane permeability of potassium is further increase, causing hyperpolarization.
Absolute refractory period (sodium channels inactivate)
-sodium channels remain inactive until membrane potential is repolarized (deinactivated) to about -65mv. This is required to reset the voltage gated sodium channels.
-sodium channels go from inactive and closed to active and closed
Relative refractory period (potassium channels close)
-during this period, the voltage gated sodium channels are active but closed. But the delayed refractory potassium channels are still open, and take some time to close.
-prolongs hyperpolarization
-during this period it is more difficult (but not impossible) to initiate further action potential.
What causes the intial membrane depolarization?
“inherited” from adjacent axonal region
a depolarization in one part of an axon leads to a small depolarization to an adjacent part and another adjacent part.
“inherited from dendritic depolarization (due to inputs from other neurons)
if sufficient dendritic depolarization occurs, then we could get a depolarization at the axon hillock or the spike initiation zone - and that’s going to be the first place we observe an action potential in our neuron.
Physically-gated Na channels may open (e.g. stretch sensitive Na channels in skin’s sensory neurons)
literally stretched open. This leads to action potentials in the axons of these sensory neurons
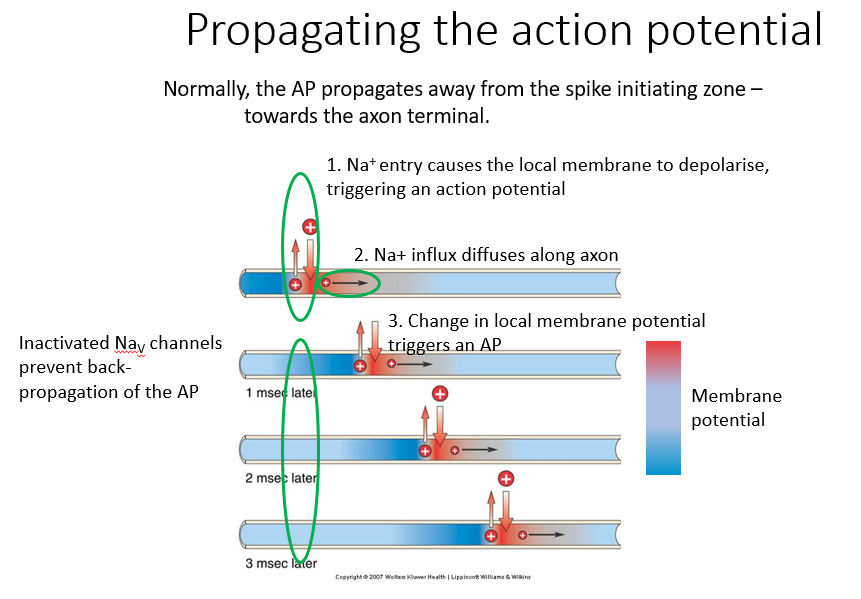
Propagating the action potential:
Normally, the AP propagates away from spike initiating zone towards axon terminal
Na+ entry causes local membrane to depolarize - triggering action potential
Excess sodium diffuses along length of axon. If sufficient sodium ions diffuse, then we’ll get a depolarization in an adjacent region of the axon
change in local membrane potential triggers an action potential. So we get this propagation along the axon.
IMAGE: The blue region is inactivated Na channels - which prevents back-propagation of action potentials.
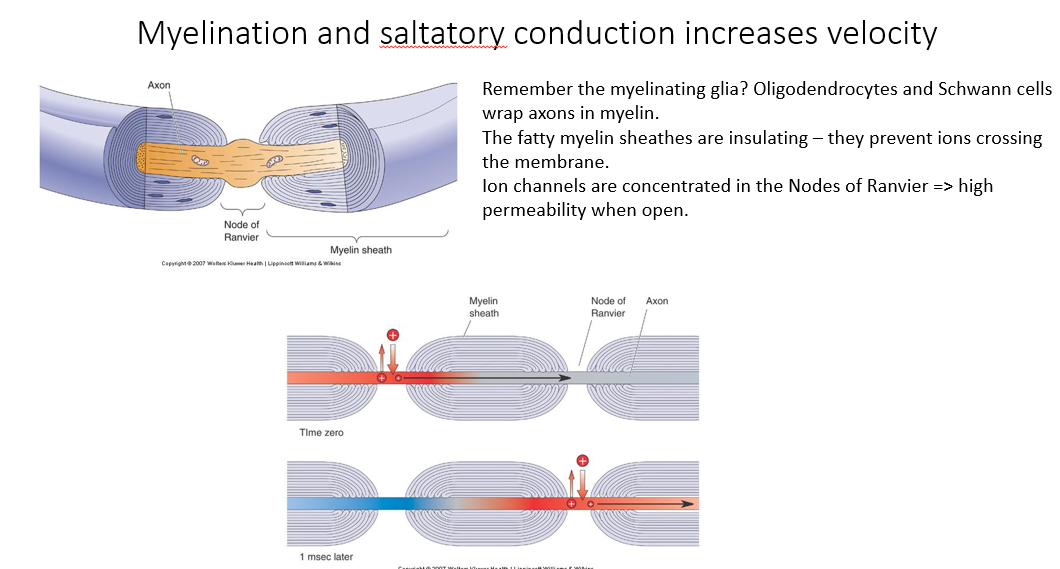
Myelination:
What does it do?
What produces it?
What are nodes of Ranvir?
What is saltatory conduction?
Myelination provide reliable and rapid conduction.
produced by the myelinating glia
insulate the axons or insulate the membrane separating the intracellular and the extracellular space
Node of Ranvir:
the gaps between myelination
regions which have high channel density
intermediate (insulated) regions have very low channel density because it’s very difficult for ions to move from the intracellular space across the myelin sheath into the extracellular space
Saltatory conduction:
Essentially, we’re getting action potentials leaping from on node of Ranvir to another.
This means that the internal diffusion of sodium ions between the nodes has to be sufficient to cause depolarization at the adjacent node to actually cause an action potential (because if there is insufficient diffusion, we won’t get action potential conduction).
Without the myelin sheath, we would get lots of individual action potentials at many places. But this would slow it down a lot.
If the myelin is in large spacings, it might be insufficiently depolarized to actually cross the threshold at the adjacent nodes of Ranvir.
How do these things affect conduction velocity?
Myelination:
Thickness:
Temperature:
Myelin - more myelin is usually going to lead to faster conduction velocities
Thickness - axon thickness affects conduction velocity
Temperature - higher temp generally increases conduction velocity. But not much in humans, because our blood supply keeps our nerves at a relative temperature.
What happens when intravenous injection of KCl occurs?
What happens in the brain?
K+ ions leave the blood and enter the extracellular space around the body.
This depolarizes the resting membrane potential (membrane potential increases)
usually high K+ concentration inside the cell, so increasing extracellular K+ concentration decreases the gradient between inside and outside the cell. SO inside of the cell becomes less negative.
But this can prevent the cell from resetting/ repolarizing. For cardiac cells, this can prevent the heart from beating.
In practice, the brain is protected from such rises in K+ because:
blood brain barrier (prevents free movement of K+ ions from bloodstream into extracellular space of brain
Astrocytic buffering (astrocytic buffering should be able to spatially move potassium around to prevent excessive build up in extracellular potassium concentration)