Laboratory animal sciences Course
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
78 Terms
Animal models: Choices and Consequences
---------
what are animal models still considered useful?
the complexity of living organisms at some point requires research and testing on animals, we still rely on the full imunological response of animals, also to understand the side-effects (require animal models to put a a new drug on the market)
what is required in order to create an animal model and what are the different types?
need to be able to understand the mechanism of action and have at least one aspect that you understand to study biology or the pathological process. there are subdivisions: disease models (induced-experimental disease model including obesity, tumor growth) spontaneous-genetic disease models (nude mice, hypertensive rats) transgenetic dieaseae (knock-out mice, tissue selective)
Neutral models: use healthy animals from all species
do you need in-bred or out-bred animals?
inbred strains are produced by more than 20 generations of brother x sister mating, the risk is genetic drift due to a accumulation of new mutations but can create genetic homogeneity
outbred strains have as much genetic variability as possible, the amount of genetic varieation depends on the breeding history of the particular colony
inbred strain are easy to control but outbred strains are difficult and you need to track the genetic variability
you should use inbred strains because they have a certain genotype, this controls for the differences between experimental group, and you can use less animals, higher statistical reproducability
is it better to have inbred or outbred populations in toxicology?
inbred strains are more stable, more uniform, repeatable, and better defined than the genetically undefined outbred stocks used in most toxicology testing. umore than one strain can be used in a factorial experimental design without increasing the total number of animals.
what are diversity outbreds?
they mnaged to have a strain of mice that has genetic variability that is consistent so that over time you could repeate the experiment and have the exact same genetic variability. you avoid genetic differences but still requires large groups, is expensive. an advantage is that you can do higher resolution mapping of genes and locate the genetic basis of certain effects that you are trying to model
how do we choose the right animal model?
ivestigate the species, life stage, genetic modification, read literature, contact peers, and make a well defined defintion of what you need in your study. go with the "lowest" species if you can use fish do so (the ethical committee will consider it this way).
why are mice and rats an ideal species?
anatomy and physiology are similar to humans, small and easy to maintain, good reproductive performance
how do we check the validity of the model
Face validity (resemblance in symptoms and signs)
Predictive validity (resemblance in reaction to current treatment)
construct validity (resemblance in origin/mechanism)
where do you get your animals from?
dutch law requires you to obtain by breeding or buy from an accepted licensed supplier. there are exemptions for fish, pigeons, goats, turkeys, chickens, horses, cows, sheep, pigs. just needs to be repeated
what are the differences between B6J and B6N mice?
need to understand the differences. there is a high health status, they are well characterized, most published kind, extensive phenotypic data, consistent data reproducibility.
what is genetic drift?
the tendancies for genes to evolve even in the abscense of selective forces, can happen at random, signle base changes, mistake in miosis, DNA repair, mutations can accumulate. the colony size impacts this. in a large colony the effects of a mutation will be smaller than in a small colony (small colonies are more vulnerable.
how do substrains develop?
through genetic drift. this can happen very quickly and can be invisible. colonies seperated by 20 or more generations are at risk of have genotypic or phenotypic results
how do we minimize genetic drift
not isolating aggressive animals, amintain pedigree lines and detailed colony records, watch for phenotypic changes, refresh breeders frequency, avoid selection pressure, verif genetic backgrounds with genome scanning, cryopreserve unique strains
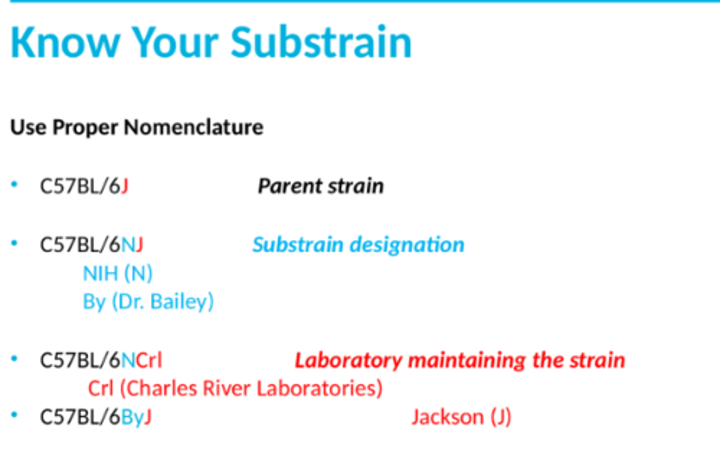
sub-strain name definitions
letter after the 6 is the parent strain, second letter is the sub-strain designation, finally the laboratory that is maintained by the labs. both control and experimental animals need to be from the same sub-strain because they can differ genetically through single nucleotide polymorphisms(SNPs), insertions and deletions (indels), cop number variation(CNVs), and spontaneous mutations. they can also differ phenotypically through metabolism, immunology, neurobiology, and more.
The ethics of animal experimentation
--------
false dilemmas
by pinning two sides against each other it ignores the complexities of the arguments

what is ethics?
the study of right a wrong statments (dilemmas). asks questions around who benefits and who is harmed? is it fair for another species to suffer for the benefit of human. can be:
Descriptive (who says what and with what grounds?)
Normative (making an argument for the right/wrong statements) Evaluative (can we make a tool to weigh these dilemmas, objectivization)
what are the two perspectives of ethics?
Antropocentrism: human centered, about what we value in human beings (animals may have only instrumental value.
Zoocentrism: animals have intrinsic values
(neurolaw and the ethics of animal trauma and consciousness)
-------
nee tenzij
prohibited-unless
what is the ethical policy of animal testing
it is a societal and moral dilemma. testing on animals is always morally problematic but not necessarily morally rejectable. testing is prohibited unless the institutions say otherwise
what are the five freedoms of animal testing and what does the law say about these freedoms?
1. freedom from hunger and thirst, from discomfort, pain, injury or disease, to express normal behavior, and from fear and distress
an animal test according to the law is an experiment on animals the infringes on these five freedoms
not all animals are considered animals by law (grasshopper being an animal but also an insect)
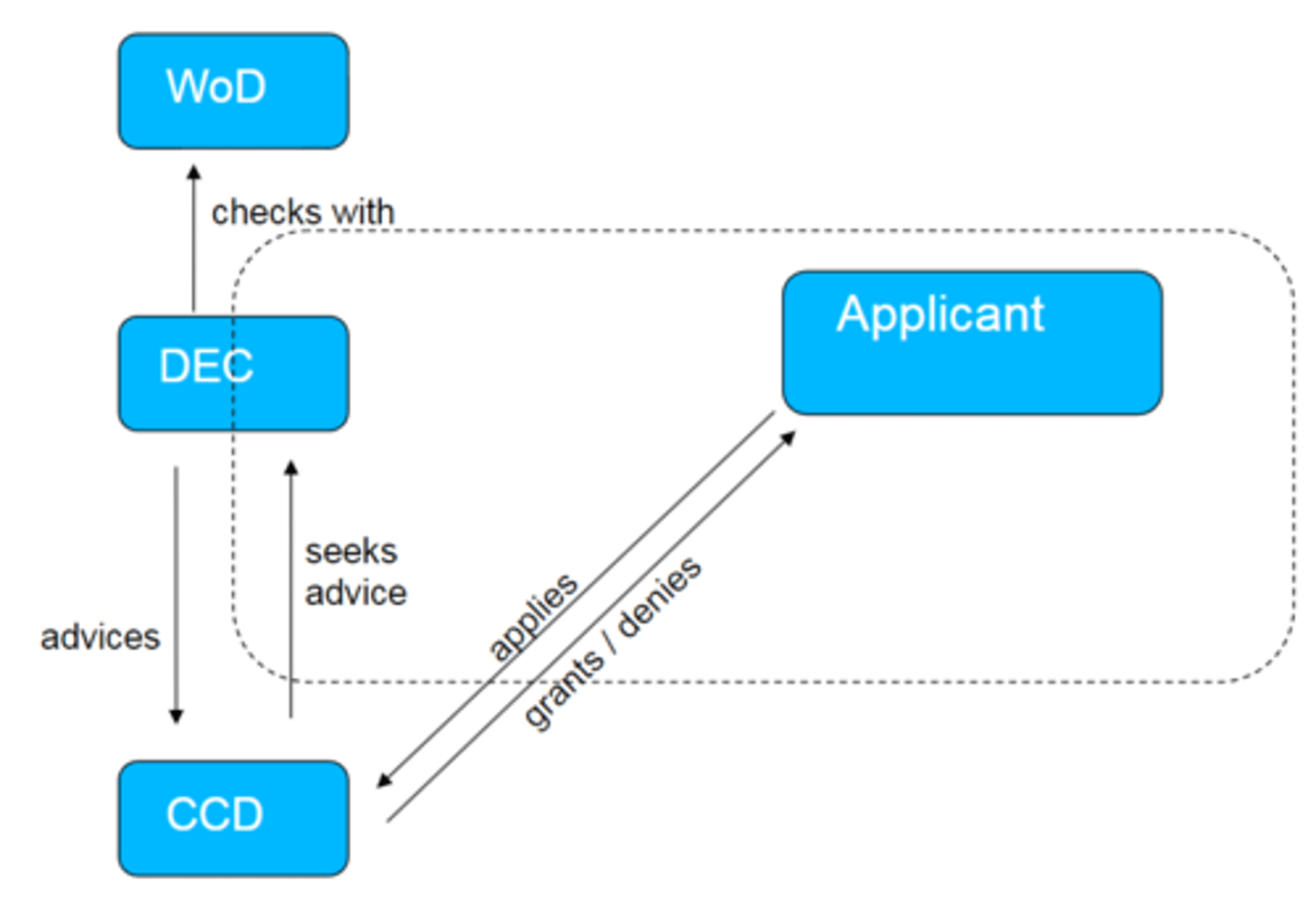
what is the structure in which experiments are made?
WoD: law on animal testing
DEC: committee of animal ethics
CCD: central committee on animal testing
Dotted line is the institution
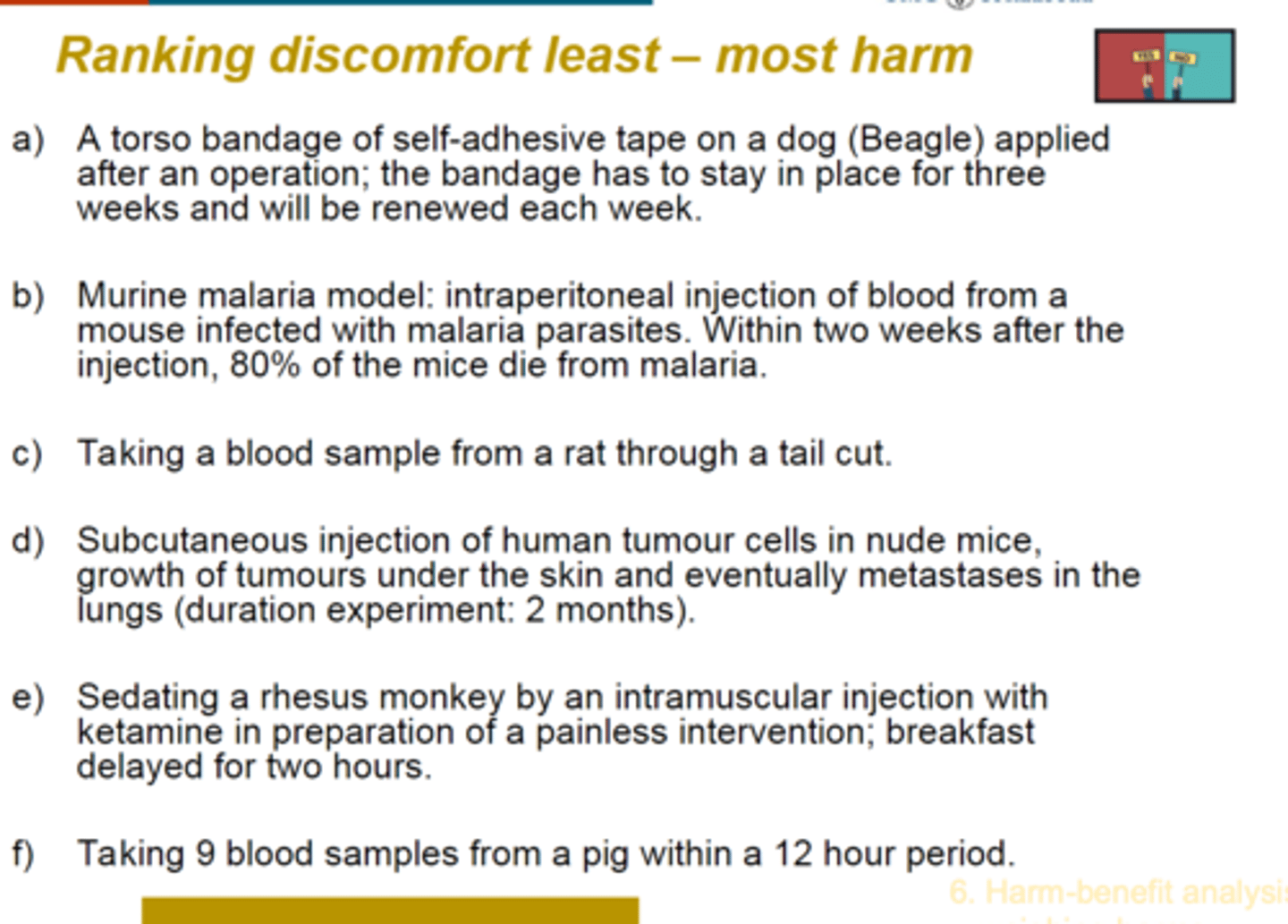
ranking of discomfort
C: minimally invasive, fast healing, minimum sensory receptors,
E: stress from seperation from group, painful waking up from ketamine
A: painful of taking bandage off, repition and duration
D: after injection for a while there isnt anything going on, only after a long period of time ther will be pain but only at the end, the animals are sacrificed in the end
B: 80% of the animals die from pain and suffering
what is the total about of discomfort determined by?
severity of direct discomfort (the procedure) + the severity of contingent discomfort (housing, transport,etc) X the frequency whith which discomfort occurs + the duration of discomfort = cumulative suffering
what is the weighing of the weighing procedure?
Mild:
Moderate: short term moderate pain or long lasting mild pain, moderate impairment of the well-being
Severe: short term severe pain, long lasting moderate pain, severe impairment
Non-recovery: must be performed under complete anesthesia without recovery
what is the weighing procedure?
everything done to the animal during the project, set on the most severe effects that can be expected after application of three Rs, not just expectation, also monitoring and final assessment of actual harm
when is animal testing never allowed?
the chance of the experiment fails is higher than needed
Experimental Design and Statistics
------
what is the message of statistical significance?
statistical significance is not the same thing as clinical relevance
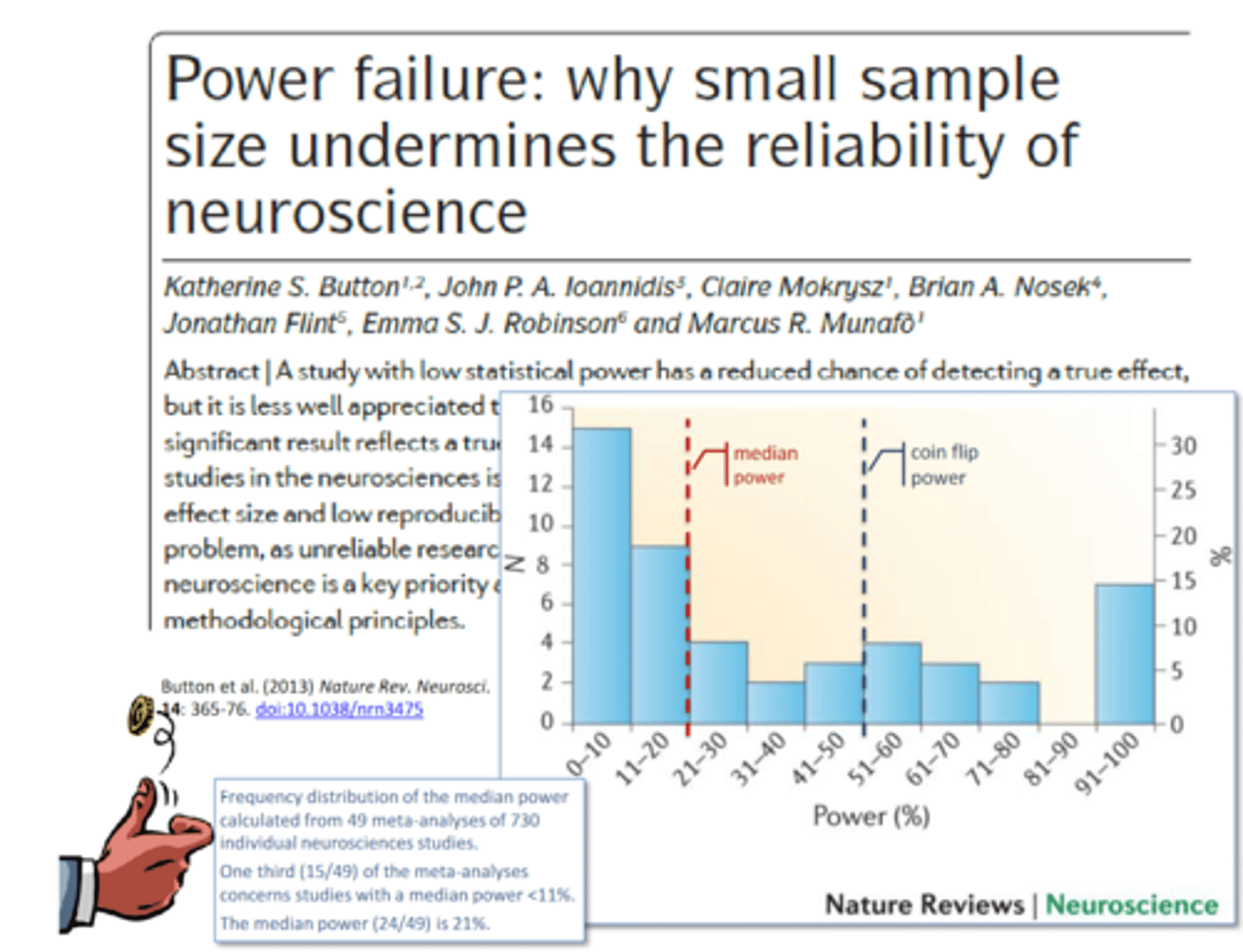
what is the replication crisis due too?
replication crisis is mainly due to too small a sample size
what are the key steps in experimental design
1. Start out with a well-defined hypothesis and research question
2. Design the appropriate control group(s)
3. Determine appropriate sample size.
4. Randomly allocate experimental units totreatments
5. Control and reduce variability through blocking(stratification) and factorial experiments
when should you perform a t-test?
one indepedatn variable which is caregorical and one dependent variable which is numerical you should perform a t-test
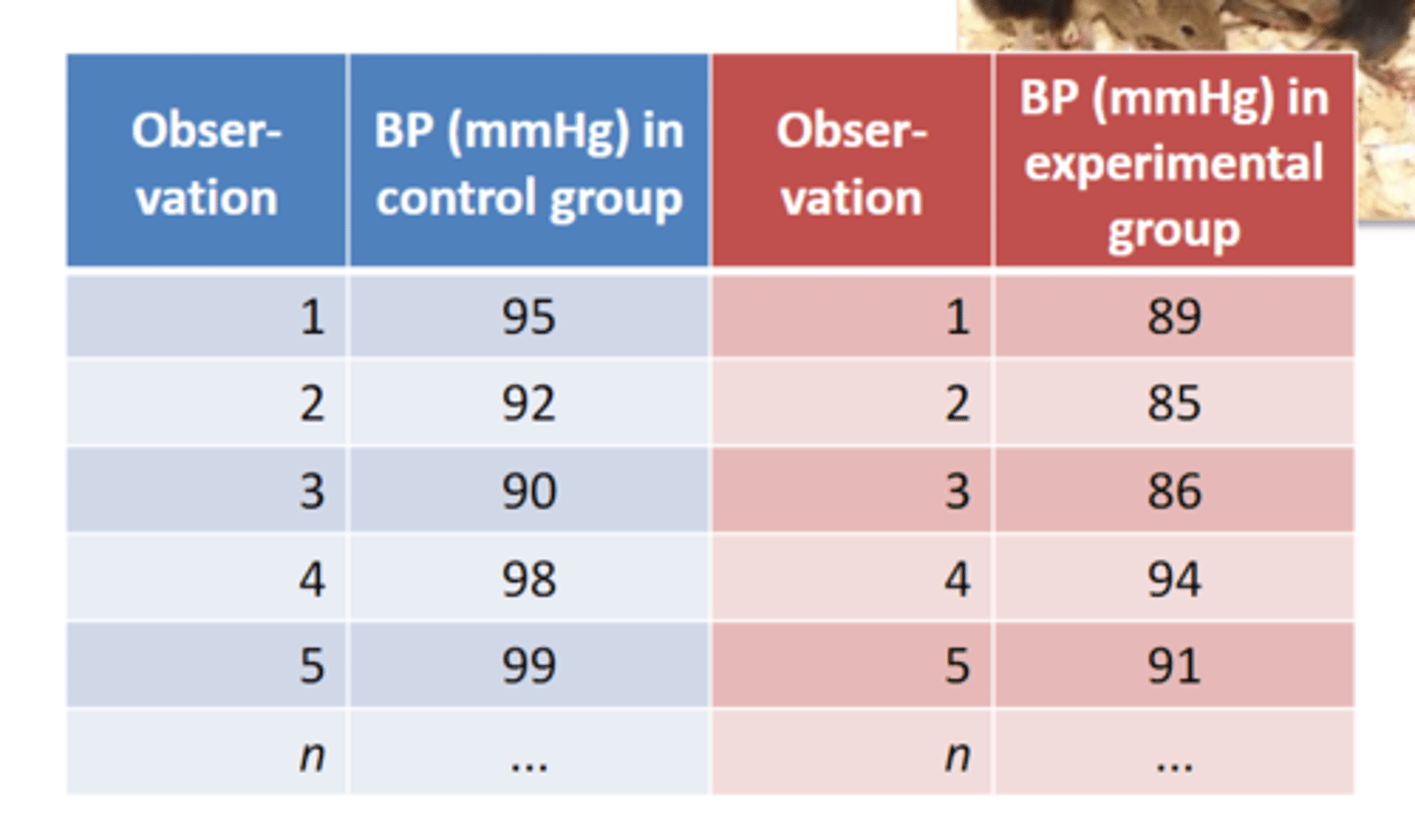
why should we randomize?
always randomize, you can never exclude confounding factors between groups
what does the p-value tell us
null hypothesis is the hypothesis of no effect, P values tell us the probability that your data supports your null hypothesis, lowest level of power is 80%
what are the effects of variability on power
if you reduce variability (same sex, same breed, same size etc) this increases power
what is the dilemma between significance level and type two error in statistics?
you cant reduce alpha (significance level) and reduce the type two error at the same time
BEANS
A= alpha and is they type-1 error(false positive) we dont want this more than 5% of the time
B= beta or type-11 you want no more than 20%
E= the effect size
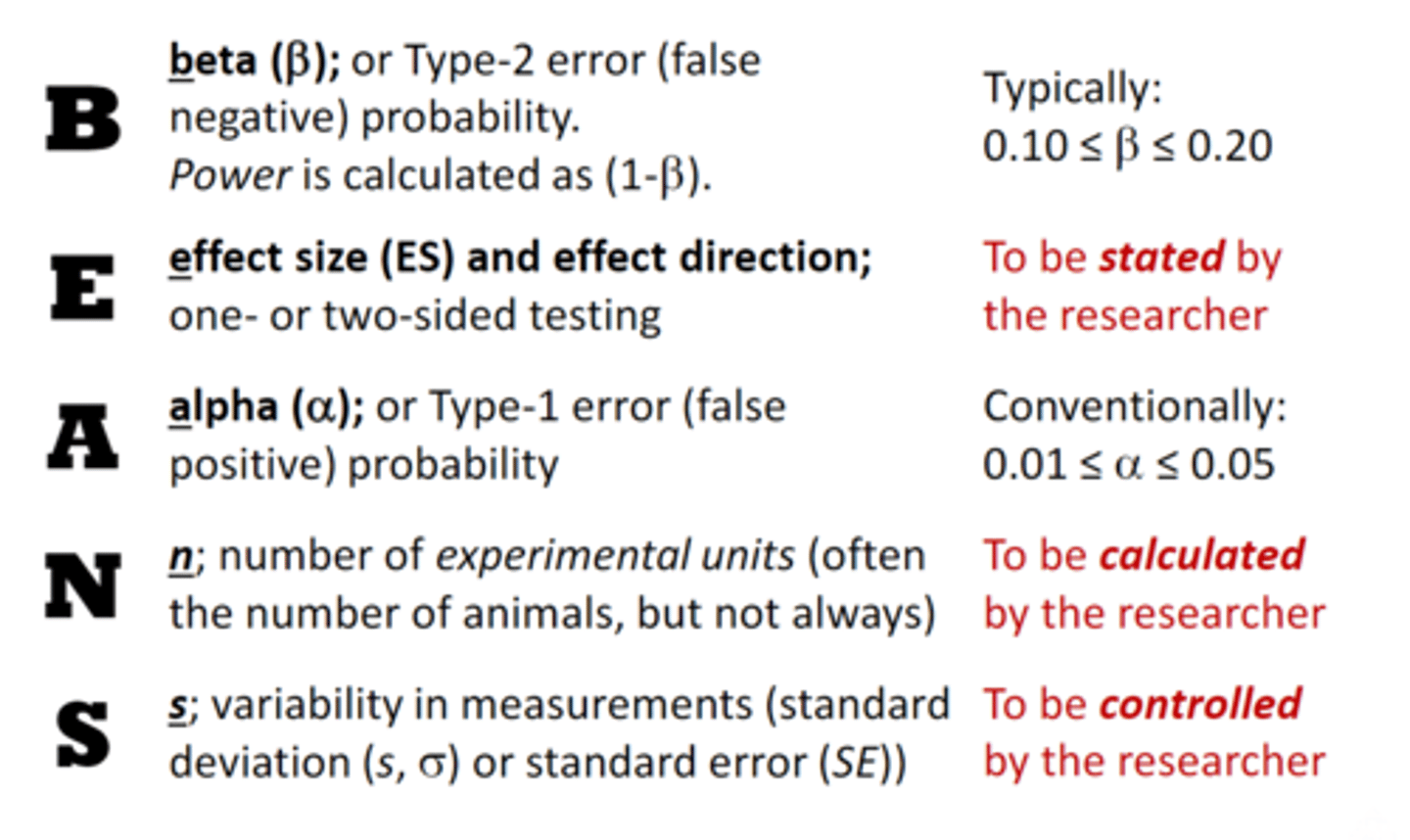
what is the difference between effect size and p-value?
effect size (how much does something differ in a different condition, gives information on how different two samples are)
P value (is the probability of finding an effect at least as extreme as the observed, it only tells you that two samples are statistically significantlly different, it does not tell you how different two samples are, the effect can be significant (p < 5) but not practically relevant)
standard deviations
there is variation and error everywhere in life, biological variation, correction, resolution of instrument, inconsistent use of protocols. Anything that you can do to reduce error will help you improve consistency
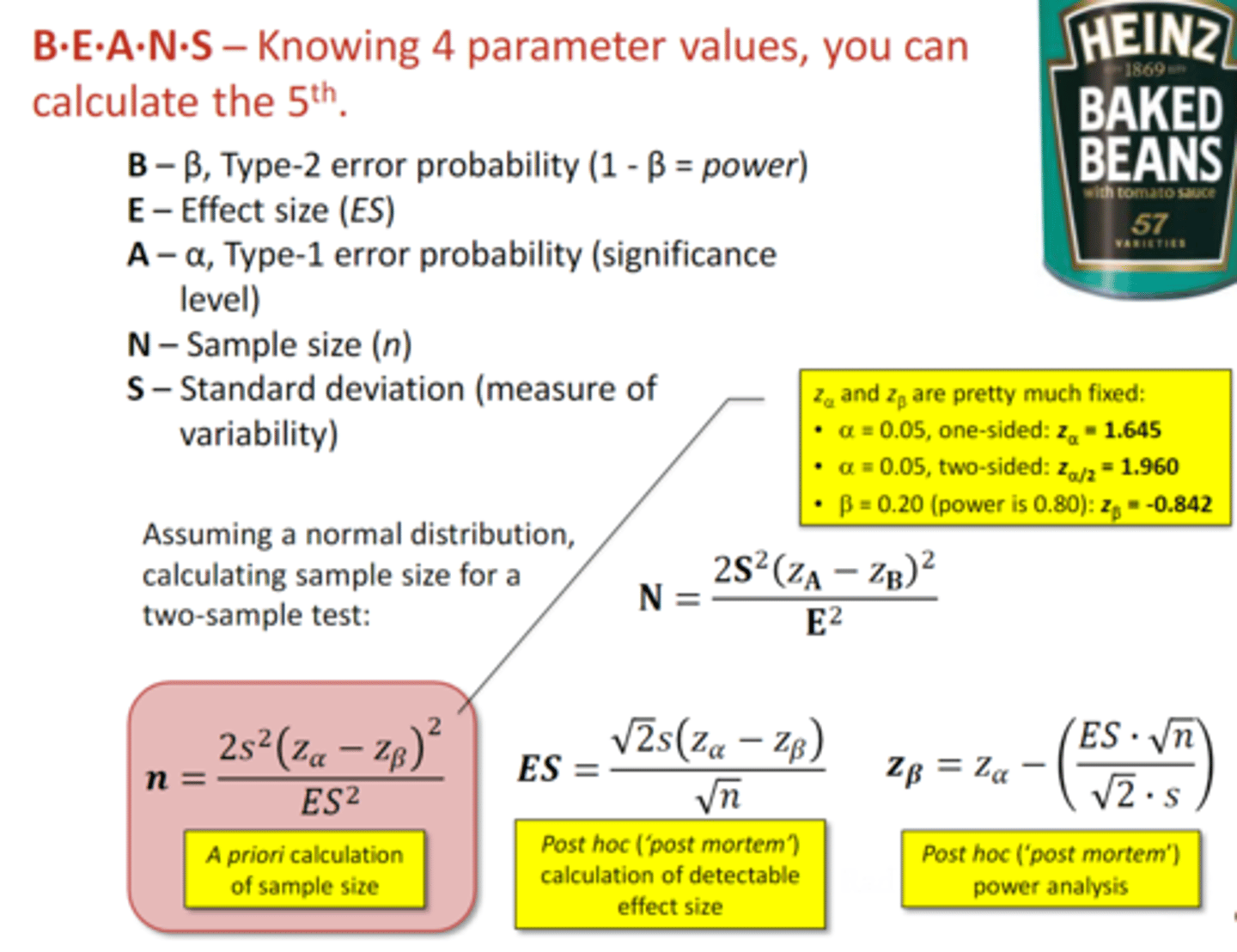
do an a priori calculation
you can calculate the sample size
3Rs- Alternatives to Animal Experiments
-------
What are the 3 Rs
Replacement: The use of alternative methods that do not include sentient beings
Reduction: a means of “lowering the number of animals used to obtain information of a given amount and precision”
Refinement: the process of minimizing the number of procedures or severity of pain that an animal must endure
what are the different motives for developing the 3Rs alternative
law, ethics, scientific, economical, public pressure
what is a well known replacement model?
In vitro systems: cell organelles, primary cell cultures, cell lines
a disadvantage is that they don't survive very long and lose their differentiation and we still have to kill animals to obtain these cells. We can share these cells with others and compare with others
stem cells are good sources.
organs and slices from large organs (such as the liver)
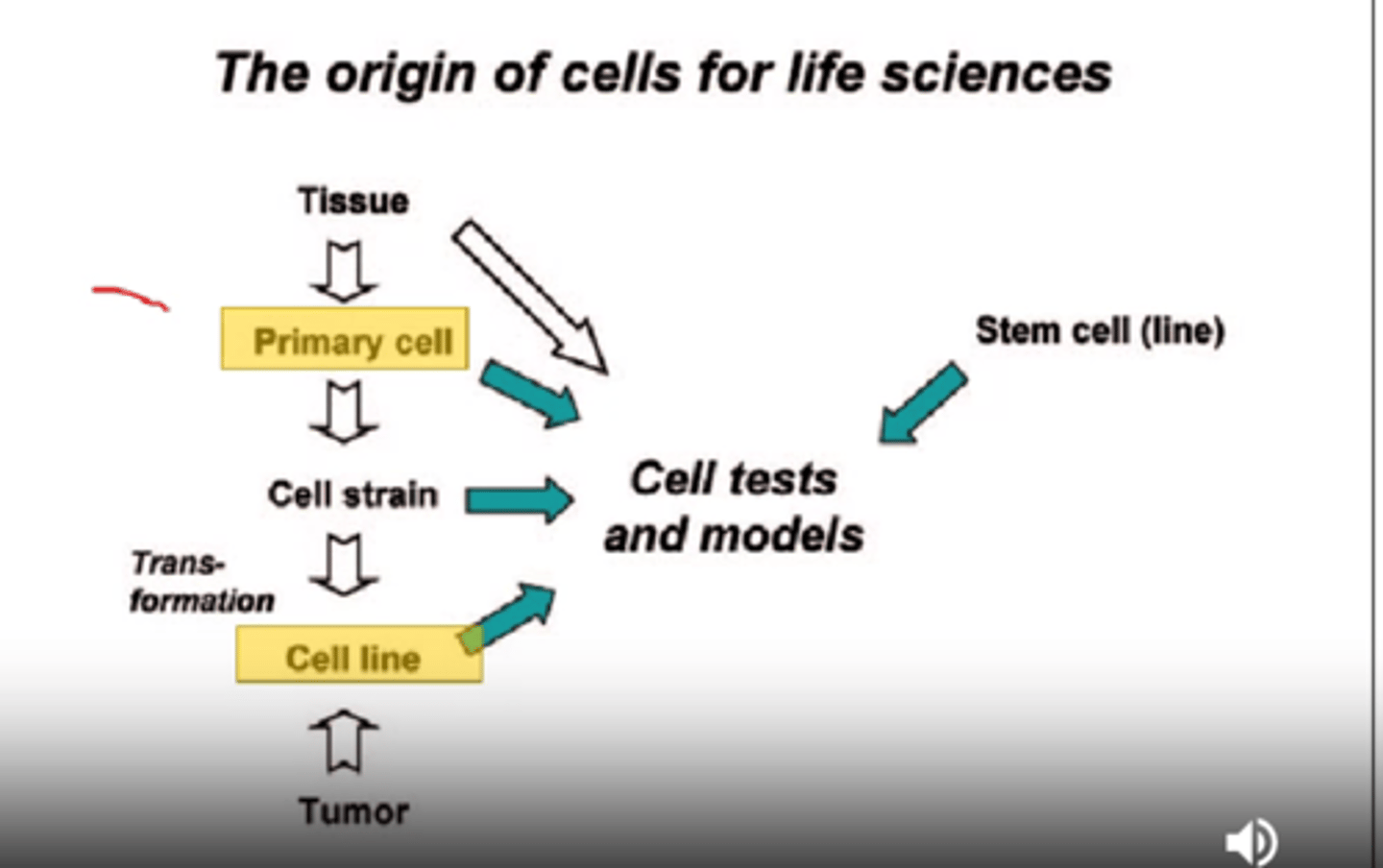
what are the advantages in vitro systems?
there are no interactions with other organs, there is increased sensativity, better interpretation, better control of conditions, increased experimental flexability (we can change to protocol), great sample capacity, and there is a smaller quantity of the substance needed
what are the disadvantages in vitro systems?
there are no interactions with other organs, need information on bio-kinetics, may have been changed by bio-transfer, possible property changes due to the environmental changes (we always have to validate cell systems), difficult extrapolation onto animal models and human models
what are the disadvantages in vitro systems from classical cell and tissue culture?
the evironment is static leading to a changing of the medium multiple times a week, the rich medium gives a shock to the cells, the medium is undefined (fetal bovine serum), the cells are grown in a two-dimension way
what are some replacements for animal studies?
in vitro techniques, audio-visual aids, slaughterhouse left-overs, invertebrates (besides octopus and squids), human materials, live human experiments (non-invasive or cause little harm), fertilized chicken egg membrane, modern techniques (computer modeling/simulation)
what is 2nd R-Reduction?
use the minimum number of animals for a study, sharing the animals to make better use, choice of animal model, education and training, longitudinal measurements, ethical verification, experimental set up (standardization/statistics), testing strategies using other techniques before going to an animal
what are examples of the 3rd R-refinement?
animal care, studies must be performed by skilled persons, analgesia, anesthesia, and euthanasia, non-invasive procedures when possible, and animal behavior must be studied to better understand their behaviors
Microbiology and hygine
------
what are the effects of different viruses on mice
viruses and disease can change the outcome of your results by a large margin
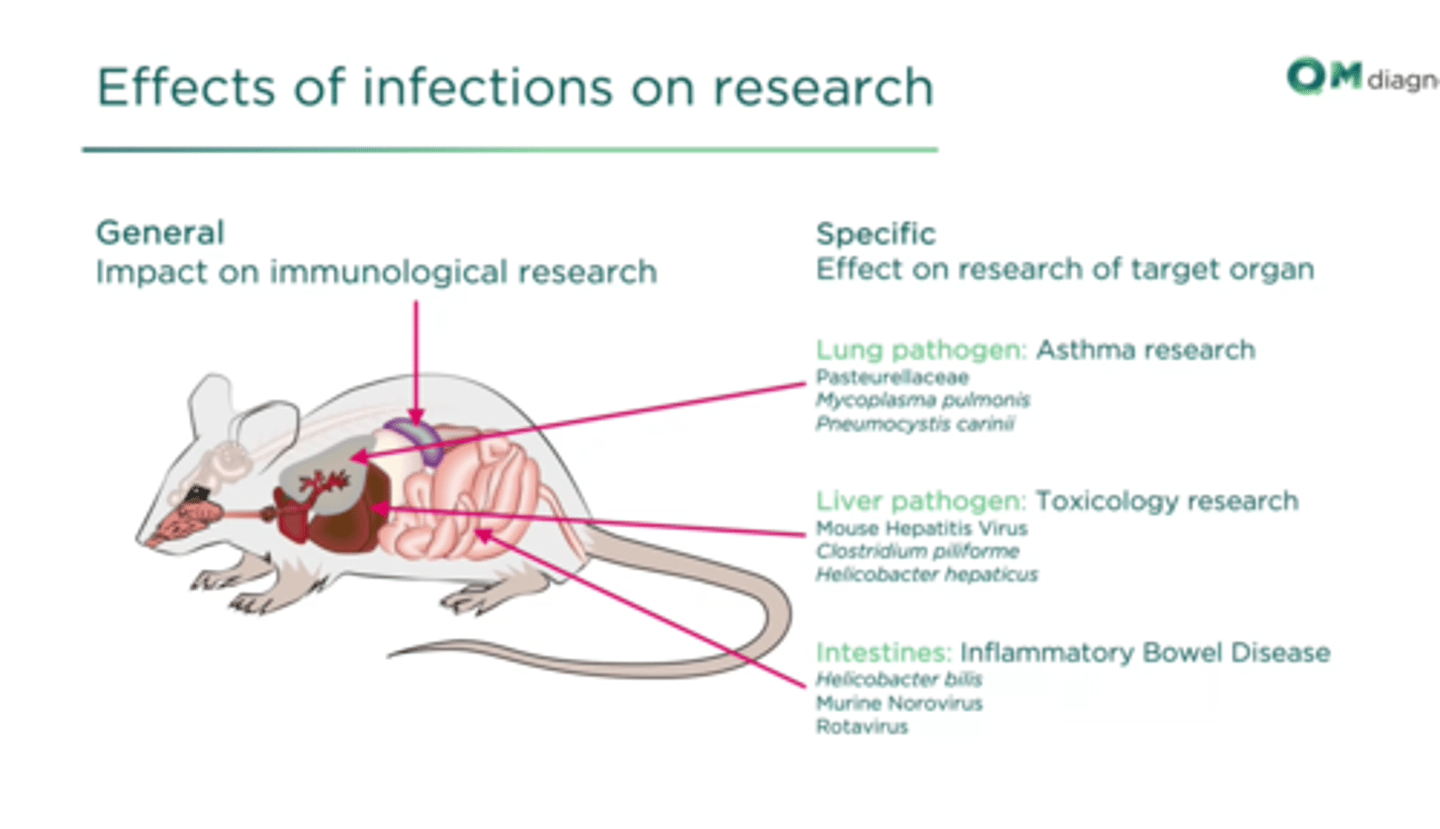
helicobacter hapaticus
it interfears with the research: colitis in immunodeficient animals, impact on oncology studies (tumors in liver), immune modulation, rectal prolapse
is common in around 20% of labs,
what are some different animal welfare diseases
Syphacia (pinworm): causes discomfort and stress, itching of the anus
Mites: live in fur and skin, suck blood resulting in anemia, burrow into skin and results in itching, when itching animal can break barrier of skin and cause infection
Mouse Norovirus: causes diarrhea, was very prevalent, can result in malnutrition
Pneumcystis: can result in pneumonia, most present in immune deficit animals, is a fungus that lives in the lower lungs
what are the consequences on infection
increased variabiliyy=low stat power=bigger group size, may need to execute the whole facility, it is a large waste of animal life
what are the different health standards
conventional: positive for one or more pathogens normally not accepted
Specific pathogen free: negative for all specified pathogends
Specific oppertunistic pathogen free: negative for all specified pathogens and opportunists, breeder standard for immune deficit mice
gnotobiotic/defined flora: completely known flora
germ free/acenic: no microorganisms present (sterile), used in association studies
what are some different controllable barrier components?
Ventilation: HEPA filtration
Water: sterile filtered, UV light, acidification, chlorination
Diet: radiated, autoclaved
equipment: autoclaves, UV light, radiated, ethylene oxide
Housing: animal handling procedures, cleaning procedures
the risk of introducing an infection is directly correlated with the number of times a risky-operation is performed, we can minimize this by lowering the umber of people allowed in facility, minimize the number of animals imported from unqualified sources, and the biologicals imported from unqualified sources
what are the main consequences of a compromised barrier?
it can be very resource demanding and financially expensive, a reduction in efficiency and flexibility for employees and users, necessary to ensure the proper function of the animals (more journals want a detailed information about health), consequences of a compromised barrier, loss of resources, time, delay in grants, there can be a loss of animals, and loss of reputation
Anesthesia and Euthanasia
--------
what is the most use anesthetic in the Netherlands
90-95% of anesthetic use is isoflurane because it is easy to use and easy to adjust, it also has the least amount of irritant effects. must need to use a facemask and cannot be used with a pregnant surgeon
what are some variables that could influence the temperature check
has the temp. probe moved, what is the measurement of the anesthetic, which anesthetic is uses (inhalant vs injection), how the induction of anesthesia went (induction with heating map)
what are is the response to a low O2 peripheral saturation
check the breathing and heart rate, check the sensor, supply additional oxygen, check the oxygen mask, check for damage to the brain for raspatory depression, check the level of oxygen in the tank, check the baseline o2 saturation, peripheral vasoconstriction
Principles of Analgesia
—
do animals experience pain?
the responses seem to be similar in animals (similar behavioral responses), we also know that when animals are given analgesics then the animals feel better
what is pain?
the unpleasant sensory and emotional experience with, or resembling that associated with, actual or potential tissue damage
how is pain affected by mental states?
positive and negative affective states as well as cognitive processes such as attention and memory can increase or decrease pain
Nutrition of Lab Animals
what are the different types of experimental diets?
natural ingredient diets: balanced “whole foods” diets, semi-purified diets, chemically defined diets: pure glucose, pure amino acids (not used often)
what are some measures to prevent interference by variation in diet composition?
avoid batch differences between control and test animals, avoid variations in components that are known to affect the results, describe in papers the diets used
Organoids and Organs on a Chip
—
what are some current challenges involved with organoids?
very high drug prices 9due to overall low success rate), drugs fail late in the pipeline, poor extrapolation from humans-animals/side effects
what are some solutions to the issues with organoids?
increasing screenings, more physiological animal models, 3Rs
what are organoids and what are some defining features?
an artificially grown mass of cells or tissues (any organism) that resembles key organ features. The self-renew and differentiate, you can use it to study disease and treatments, difficult to control and/or modify the preprogrammed architecture
what are some defining features of organ-on-a-chip microdevices?
control over compartmentalization, finer control over gradients, ability to expose it contents to physiological conditions
Recognition of pain, suffering, and distress in specific species
—