poisons
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
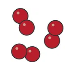
element
an element is a pure substance made up of just one type of atom.
element diagram
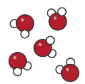
compound
a compound is a pure substance made up of more than one element that are chemically bonded together
compound diagram
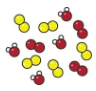
mixture
A mixture is formed when two or more elmenst or coumpounds are present without being chemically bonded
mixture diagram
water formula
H20
carbon dixoide formula
CO2
Salt formula
NaCI
similarities between modern periodic table and mendeleev’s
They both utilise periods and group to help identify elements properties and Elements with similar properties are grouped together
Differences between modern periodic table and mendeleev’s
Modern Perodic table is complete with all the elements in the table as it doesn’t have gaps and use to be orgnised by atomic mass, now organised by atomic number
Protons
1AMU, positively charged, located in nucleus
netrons
1AMU, uncharged particles, found in nucleus
electrons
1/1836, negatively charged, found in outershells around the nucleus
valance shell
The outmost electron shell of an atom. This shell determines its reactivity, or tendency to form chemical bonds with other atoms.
electron configuration
2,8,8