10. Axis formation, Neural Induction and Neural Tube Patterning
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What are the key molecular and cellular mechanisms that drive A-P/D-V patterning, induction of neural tissue and the creation of D-V pattern within the neural tube?
Key mechanisms include:
The fate and function of the Spemann-Mangold Organizer
The roles of BMP and Wnt antagonists secreted by the Organizer in neural and head induction
The "activation-transformation" model for neural tube A-P patterning
The role of posteriorizing factors (Wnts, FGFs, retinoic acid) in regulating Hox gene expression
The D-V patterning of the neural tube by opposing morphogen gradients.
Spemann-Mangold Organiser
What cells is the Spemann-Mangold Organiser composed of?
What embryonic tissues arise from the Spemann-Mangold Organizer during gastrulation?
What else is formed under the direction of the Spemann-Mangold Organiser during gastrulation?
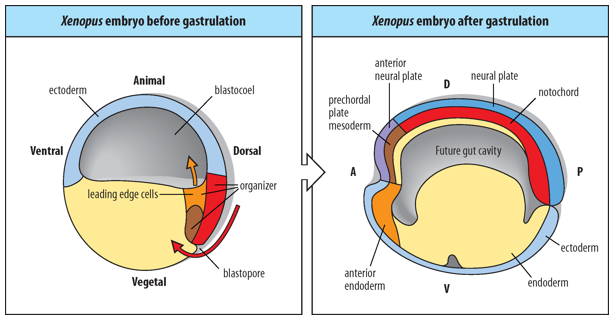
The Spemann-Mangold Organizer (also known as the dorsal blastopore lip in amphibians) is composed of mesendodermal progenitor cells.
During gastrulation, these cells give rise to 3 distinct embryonic tissues:
anterior endoderm
prechordal mesoderm (head mesoderm)
notochord (axial mesoderm).
The Anterior-Posterior Axes and Dorsal-Ventral pattern of the embryo are formed during gastrulation, under the direction of the Spemann-Mangold Organizer.
Briefly describe the classic Spemann-Mangold Organizer experiment (around 1924) and its significance.
What was done?
What was observed?
What was concluded?
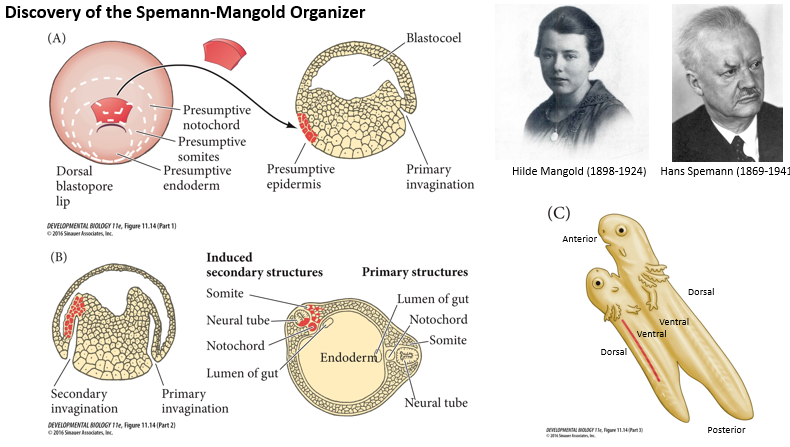
Spemann and Mangold Experiment (around 1924):
What was done?
The dorsal blastopore lip (presumptive Organiser) from an early newt gastrula (donor) was transplanted to the ventral side of another early newt gastrula (host).
What was observed?
The transplanted tissue induced a secondary invagination and the formation of a secondary, nearly complete embryonic axis in the host. This secondary axis included a second neural tube, somites, and notochord, often derived from the host's own tissues. The host embryo effectively became conjoined twins.
What was concluded?
The dorsal blastopore lip acts as an "Organiser," meaning it's capable of instructing surrounding tissues to adopt specific fates and organise into a new body axis.
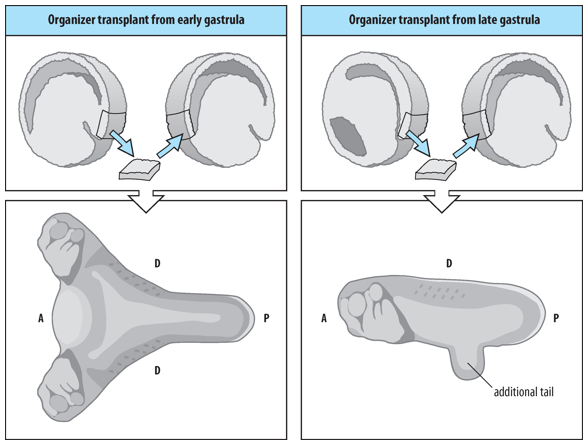
How do the inductive properties of the Organizer change as gastrulation proceeds (early Organiser/late Organiser)?
A small piece of tissue removed from the dorsal blastopore lip and transplanted to the ventral side of a host embryo can induce a secondary embryonic axis (practically a conjoined twin) in the host embryo comprising mesodermal and neural tissues and organs.
However, the inductive properties of the Organiser change during gastrulation/over time:
Early Organizer (from an early gastrula): When transplanted, it can induce a complete secondary axis, including both head and trunk structures.
Late Organizer (from a late gastrula): When transplanted, it primarily induces trunk and tail structures, but not a complete head.
This suggests that the signals produced by the Organizer change as it involutes and moves anteriorly.
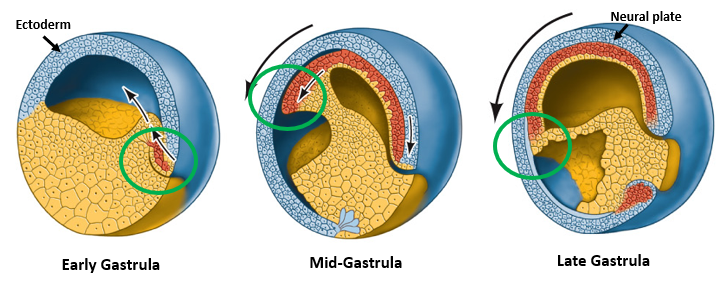
What is the fate of the Early Organizer tissue as it migrates during gastrulation?
As gastrulation proceeds, the Early Organizer tissue (which initially forms the dorsal blastopore lip) migrates into the embryo, moving anteriorly underneath the overlying ectoderm (blastocoel roof).
This movement lays down the cells that will form the anterior endoderm, prechordal plate mesoderm (important for head development), and then the notochordal mesoderm (axial mesoderm for the trunk and tail).
This migration establishes the A-P axis of the embryo.
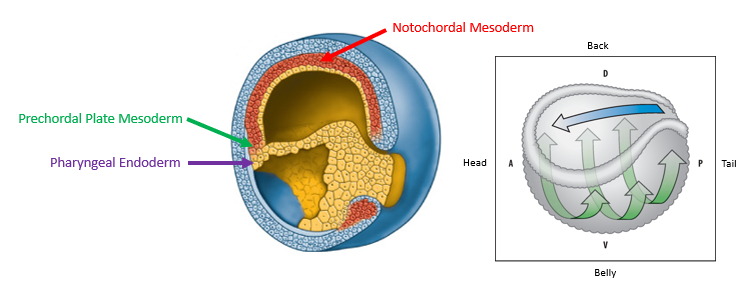
How do Organizer tissues from different stages (early, mid, late gastrula) contribute to different parts of the anterior-posterior axis?
Organizer tissues from early, mid- and late gastrulae have different fates in the embryo
Early Organizer cells contribute mainly to anterior structures: pharyngeal endoderm and prechordal plate mesoderm (head structures).
Mid-Organizer cells contribute to both anterior and more posterior axial structures.
Late Organizer cells primarily contribute to posterior axial structures: notochordal mesoderm (trunk and tail structures).
This temporal change in fate correlates with the gastrula-stages having different inducing potential because it expresses different signalling molecules over time.
What is the primary mechanism by which the Organizer exerts its inducing properties, particularly for neural induction and anterior patterning?
The primary inducing properties of the Organizer are achieved by secreting molecules that INHIBIT the activity of Wnt signaling and BMP (Bone Morphogenetic Protein) signalling pathways in the overlying ectoderm and surrounding tissues.
These pathways, if uninhibited, would promote epidermal fate (BMP) or posterior fates (Wnt).
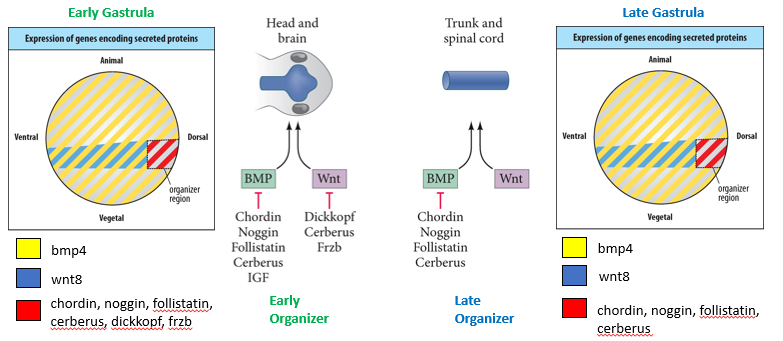
How does the expression of signalling molecules by the Organizer change over time, and how does this relate to its different inductive potentials (head vs. trunk)?
Early Gastrula Organizer:
Expresses Wnt Antagonists (e.g., Dickkopf, Cerberus, Frzb) AND BMP Antagonists (e.g., Chordin, Noggin, Follistatin, Cerberus, IGF).
This dual inhibition (low Wnt, low BMP) promotes the formation of anterior structures like the head and brain.
Mid- and Late Gastrula Organizer:
Expresses only BMP Antagonists (e.g., Chordin, Noggin, Follistatin, Cerberus). Since expression of Wnt antagonists ceases.
This condition (high Wnt, low BMP) promotes the formation of posterior structures like the trunk and spinal cord.
Anterior: no Wnt, no BMP
Posterior: high Wnt, no BMP
Dorsal: no BMP
Ventral: high BMP
Key Takeaway: The timing and specific types of secreted antagonists expressed by the Organizer tissue are crucial for patterning the anterior-posterior (head to tail) axis of the embryo.
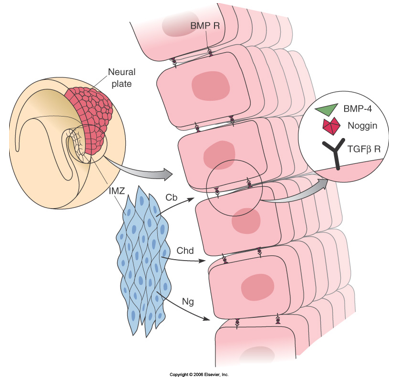
How do BMP antagonists like Noggin, Chordin, and Cerberus induce neural fate?
BMPs (e.g., BMP4) are normally expressed in the ectoderm and promote epidermal fate while inhibiting neural fate.
BMP antagonists (Noggin, Chordin, Cerberus), secreted by the Spemann-Mangold Organizer, act on ectoderm to suppress epidermal fate and induce neural fate in the neural plate. They do this by binding to BMP ligands/receptors, preventing BMP signalling in the overlying dorsal ectoderm.
This inhibition of BMP signalling allows the ectoderm to adopt its default neural fate, forming the neural plate.
Specifically for Chordin and Noggin they both bind directly to BMPs in the extracellular space, preventing BMPs from binding to their receptors - this inhibits BMP signalling. They are two of the BMP antagonists involved in trunk/spinal chord development, their specific balance and localisation contribute to the fine-tuning of the A-P axis.
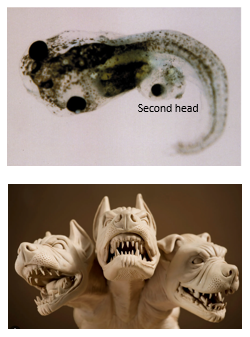
What is the specific role of Cerberus in head induction?
Cerberus, a molecule secreted by the anterior-most part of the Organizer (anterior endoderm and prechordal mesoderm), inhibits Wnt, BMP, and Nodal signaling pathways.
By inhibiting both Wnt8 and BMP4 activity in the overlying ectoderm, Cerberus promotes the anteriorisation and dorsalisation of the neural plate, leading to the induction of head structures.
The name "Cerberus" refers to the three-headed dog in Greek mythology, alluding to its ability to inhibit multiple signaling pathways.
What is the "Double Gradient Model" of embryonic axis formation generated by the Spemann-Mangold Organizer?
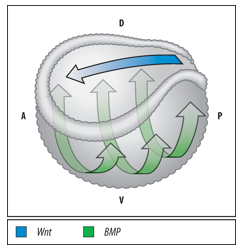
The Spemann-Mangold Organizer generates two crucial signaling gradients:
A Dorsal-Ventral gradient of BMP signaling activity throughout the embryo: BMP activity is lowest in the neural plate and dorsal axial mesoderm (dorsal region) due to high BMP antagonist activity from the Organizer.
An Anterior-Posterior gradient of Wnt signaling activity in the developing neural plate: Wnt activity is lowest in the anterior neural plate (future forebrain) due to high Wnt antagonist activity from the early Organizer, and increases towards the posterior.
These two gradients simultaneously drive A-P and D-V axis formation.
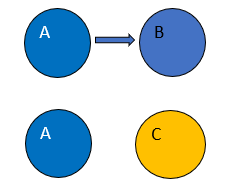
Explain the concept of "induction" in developmental biology, distinguishing it from cell-autonomous differentiation.
Induction:
A process where one group of cells (the inducer, e.g., Cell A) produces an extrinsic/non-cell-autonomous signal that acts on neighboring cells (the responder, e.g., Cell B), causing them to change their fate or differentiation pathway (e.g., Cell B becomes Cell C).
Cell-autonomous differentiation:
A process where a cell's fate is determined by intrinsic factors (e.g., asymmetrically segregated cytoplasmic determinants). For example, if Cell A divides asymmetrically, one daughter (A') might inherit a factor that maintains its fate, while the other daughter (X) lacks it (or inherits an inhibitory factor) and thus adopts a different fate X based on its own internal state.
How are the more detailed elements of anteriors-posterior pattern in the brain and spinal chord created?
What is the role of the Organiser in the Anterior-Posterior Patterning of the Neural Plate.
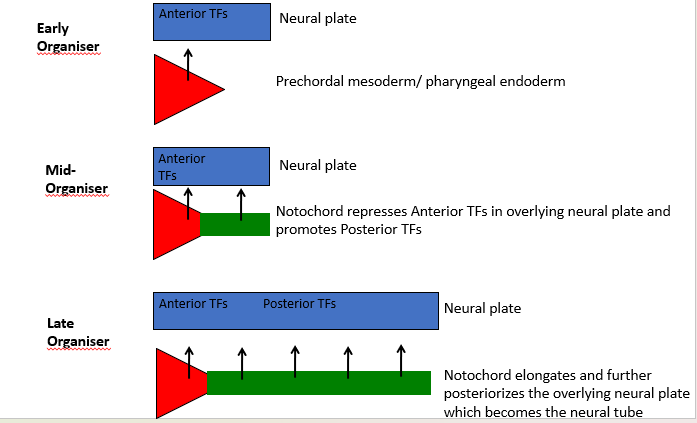
The Early Gastrula Organiser:
Wnt & BMP antagonists - Dual inhibition of Wnt & BMP signaling in the overlying neural plate.
Promotes Head and Brain (Forebrain, Midbrain, Hindbrain) formation by inducing Anterior Transcription Factors (TFs) in the neural plate. The prechordal mesoderm/pharyngeal endoderm contribute to this.
The Mid- and Late Organiser:
Only BMP antagonists - BMP inhibition without Wnt inhibition in the overlying neural plate. Suppress epidermal fate and induce neural fate.
Promotes Trunk and Spinal Cord (Cervical, Thoracic, Lumbar, Sacral, Coccygeal) formation.
The notochord represses Anterior TFs and promotes Posterior TFs in the overlying neural plate, which later becomes the neural tube. The elongating notochord further posteriorizes the neural plate.
How does the "Activation-Transformation Model" explain anterior-posterior patterning of the neural plate/tube?
Activation:
(Signal 1 - Neuralization and Forebrain specification):
1. The early Spemann-Mangold Organizer secretes Wnt antagonists and BMP antagonists.
2. Neurectoderm exposed to these dual inhibitory signals is "activated" – it adopts a neural fate and is initially specified as anterior neural tissue (prospective forebrain).
Transformation
(Signal 2 - Caudalization/Posteriorization):
3. From mid-gastrula onwards, the Organizer's secretion of Wnt antagonists attenuates (is reduced).
4. The posterior neural plate is exposed to increasing gradients of posteriorizing factors (e.g., Wnts, Retinoic Acid, FGFs) emanating from posterior sources (like the involuting mesoderm and later the tailbud).
5. These posteriorizing signals "transform" the initially anterior neural tissue, suppressing forebrain fate and inducing more posterior neural fates (midbrain, hindbrain, spinal cord) in a graded manner.
6. The neural plate elongates and forms the neural tube.
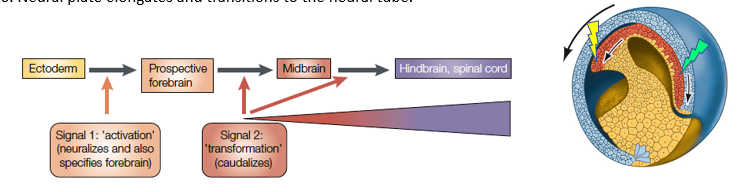
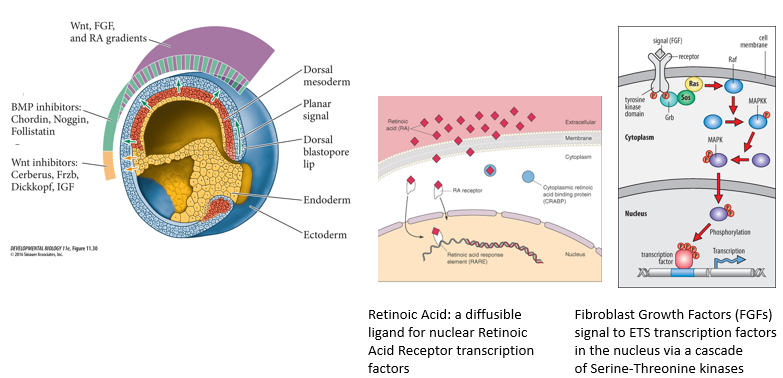
What are the 3 key posteriorizing factors involved in the process of Activation to Transformation in the "Activation-Transformation Model" of Neural plate/tube A-P patterning? How do they signal?
Key posteriorising factors include:
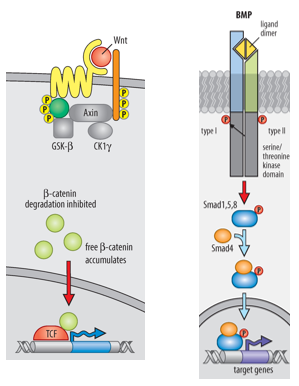
Wnts: Signal through Frizzled/LRP receptors and the β-catenin pathway.
Retinoic Acid (RA): A small, diffusible molecule derived from Vitamin A. It binds to nuclear Retinoic Acid Receptors (RARs), which are transcription factors that regulate gene expression by binding to Retinoic Acid Response Elements (RAREs).
Fibroblast Growth Factors (FGFs): Signal through FGF receptors (tyrosine kinases) and activate downstream pathways like the -MAPK/ERK cascade (cascade of serine-threonine kinases), leading to changes in ETS transcription factor activity.
These factors often form gradients from posterior to anterior.
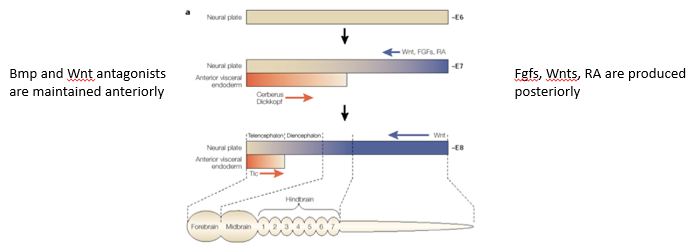
How do opposing gradients of "anteriorizing" and "posteriorizing" factors lead to regionalisation of the neural plate, particularly through Hox gene regulation?
Anterior-Posterior regionalisation is achieved by 2 opposing gradients: -
Anteriorising signals: BMP and Wnt antagonists (e.g., Cerberus, Dickkopf) are predominantly active anteriorly, promoting anterior neural fates like forebrain.
Posteriorising signals: FGFs, Wnts, and Retinoic Acid are produced more posteriorly and form gradients that decrease towards the anterior.
Transcription factors expressed in the neural plate interpret these combined signal levels.
Specific concentrations of these factors activate different Hox genes in distinct domains along the SA-P axis.
The combinatorial code of Hox gene expression then specifies the regional identity of different parts of the hindbrain and spinal cord.
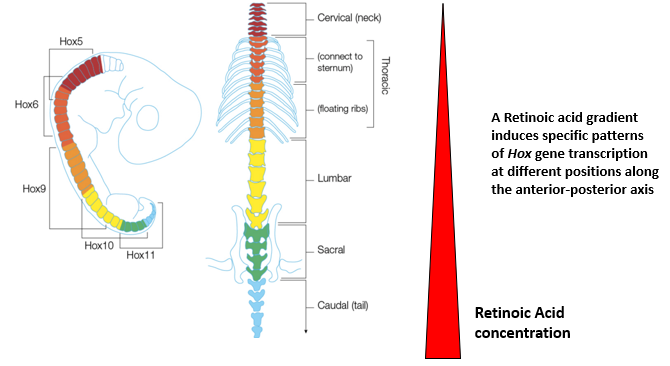
How does a Retinoic Acid (RA) gradient contribute to specifying distinct domains of the spinal cord through Hox gene expression?
A gradient of Retinoic Acid, generally highest posteriorly and decreasing anteriorly, induces specific patterns of Hox gene transcription at different positions along the A-P axis of the developing spinal cord.
Different Hox genes have different sensitivities (thresholds) to RA.
This differential Hox gene expression specifies the distinct domains of the spinal cord (e.g., cervical, thoracic, lumbar, sacral, caudal regions) and the identity of the motor neurons and other structures within those domains.
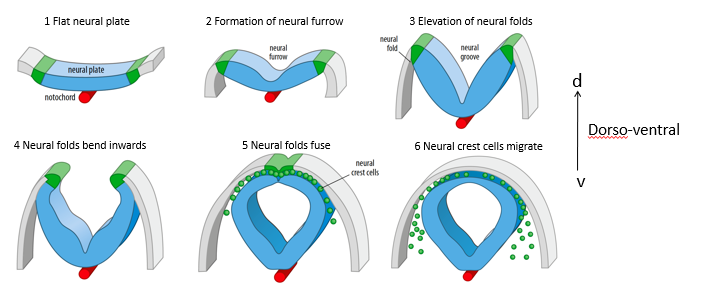
Describe the main steps of neural tube formation (neurulation).
Flat neural plate: The ectoderm overlying the notochord thickens to form the neural plate.
Formation of neural furrow: The center of the neural plate depresses, forming a neural furrow.
Elevation of neural folds: The edges of the neural plate (neural folds) elevate.
Neural folds bend inwards: The neural folds move towards each other over the midline.
Neural folds fuse: The neural folds meet and fuse at the dorsal midline, forming the hollow neural tube. The overlying ectoderm also fuses to become the epidermis.
Neural crest cells migrate: Cells at the crest of the neural folds delaminate and migrate away to form various derivatives (e.g., peripheral nervous system, melanocytes).
This is dorso-ventral.
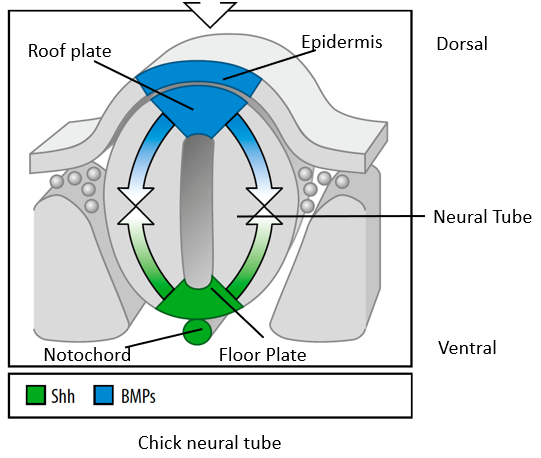
How is the vertebrate neural tube patterned along its dorso-ventral (D-V) axis?
The neural tube is polarized along its D-V axis by 2 opposing signaling systems:
TGF-β ligands (mainly BMPs) secreted by the dorsal Roof Plate induce dorsal neuronal fates (e.g., sensory relay interneurons).
Sonic Hedgehog (Shh) secreted by the ventral Floor Plate and the notochord induces ventral neuronal fates (e.g., motor neurons, ventral interneurons).
BMP signalling from the epidermis specifies the roof plate, BMPs from the roof plate pattern the dorsal half of the neural tube.
Shh signalling form the notochord specifies the ventral floor plate, Shh from the floor plate patterns the ventral half of the neural tube.
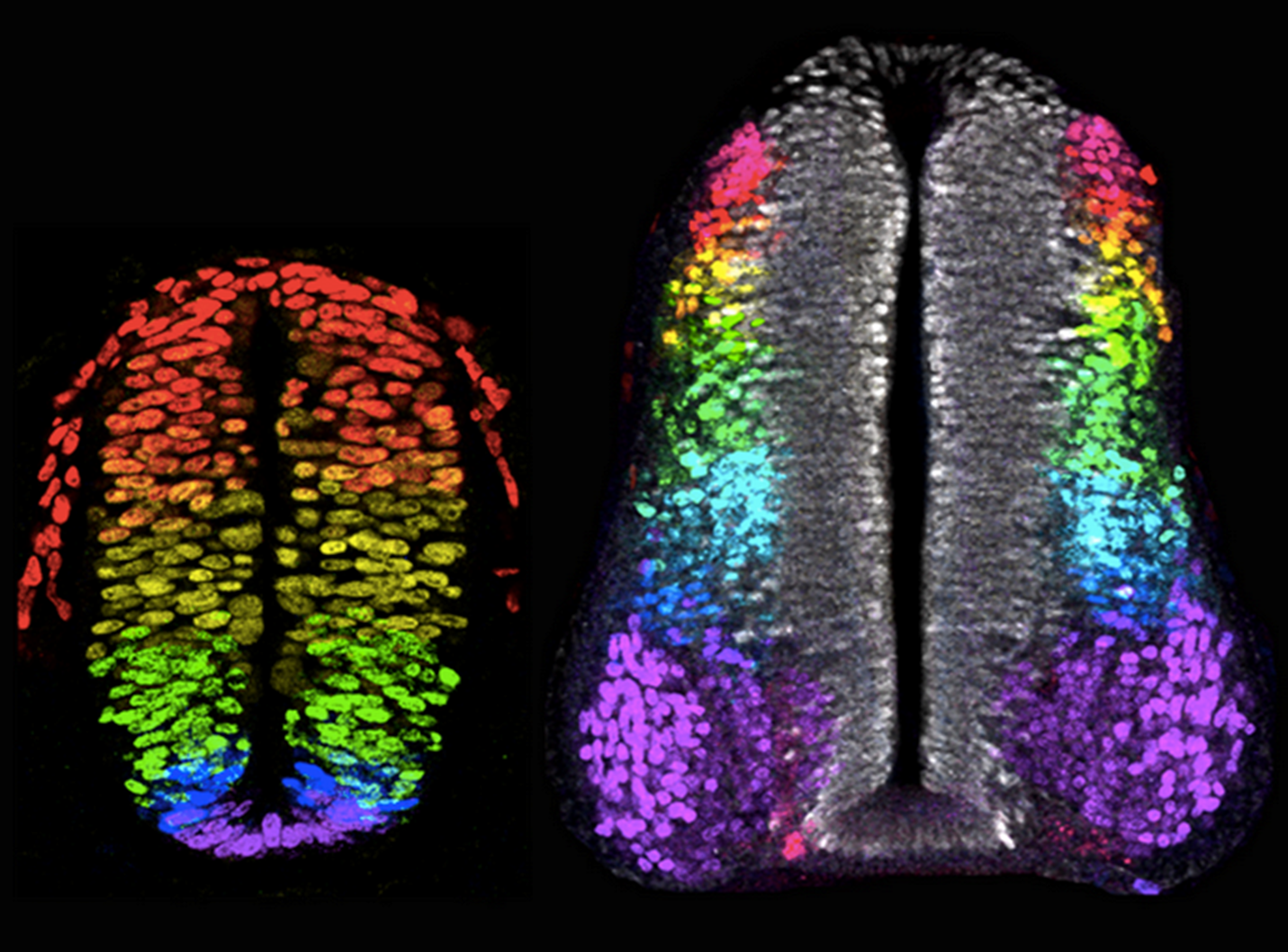
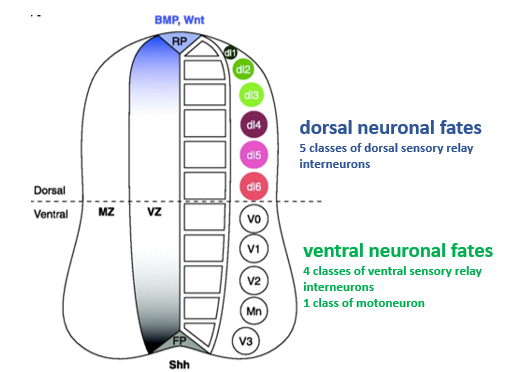
How do different concentrations of Shh and different BMP family members lead to the specification of diverse neuronal subtypes along the D-V axis of the neural tube?
Ventral neural tube:
A gradient of Shh protein, highest ventrally near the floor plate and decreasing dorsally, determines neural progenitor fate.
This is by different concentrations of Shh regulating (activating or repressing) specific homeodomain transcription factors (e.g., Pax7, Pax6 and Nkx6.1) in distinct progenitor domains.
This is done in a Shh-concentration-dependent manner. "Shh morphogen gradient" establishes multiple classes of ventral interneurons and motor neurons.
There are 4 classes of ventral sensory relay interneurons and 1 class of motorneuron.
Dorsal neural tube:
Different members of the BMP family (and Wnts) are expressed in different domains in and around the roof plate.
These likely act in combination to induce different classes of dorsal sensory relay interneurons. There are 5 classes of dorsal sensory relay interneurons.
Explanation:
Bmp & Shh induce distinct domains of transcription factor expression in neural progenitor domains along the D-V axis of the early neural tube. These transcription factors define distinct neural identities that are realised when progenitors terminally differentiate into neurons a few days later.
What is the general principle of axial patterning illustrated by neural tube development?
Axial patterning (both A-P and D-V) is often achieved by gradients or stepwise differences in competing or antagonistic intercellular signals. These signals emanate from localized sources (e.g., Organizer, floor plate, roof plate) and create a range of different cell identities in the intervening tissues by activating distinct sets of transcription factors in a position-dependent manner. These transcription factors then define the unique characteristics of cells in different regions.