O level Chemistry
1/320
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
321 Terms
Define mass number/nucleon number
the total number of protons and neutrons in the nucleus of an atom
SI unit for concentration
g /dm3 or mol/dm3
State the distinguishing properties of solids, liquids and gases
Solid has a fixed shape and a fixed volume.
Solid cannot be compressed.
Solids have a high density.
Liquid has a fixed volume but no fixed shape.
Liquids can be slightly compressed. large pressure is required to compress them.
Liquids have lesser densities than solids.
Gases have neither a fixed shape nor a fixed volume.
Gases can be compressed easily.
Gases have the least density among the three.
Describe the structures of solids, liquids and gases in terms of particle separation, arrangement and motion
The force of attraction between the particles in a solid is very strong. Thus, The space between the particles of solids is negligible.
Intermolecular forces of attraction are weaker than solids. Thus, They have considerable space between the particles.
Intermolecular forces of attraction are the weakest. Thus, The space between the gas particles is large.
Describe and explain changes of state (melting, boiling, evaporating, freezing and condensing) in terms of kinetic particle theory
when a solid is heated, the increase in KE makes the particles vibrate faster. This causes the solid to expand.
Eventually, the forces of attraction weaken, and the regular pattern of the structure breaks down. The solid has melted into a liquid.
When a liquid is heated, the increase in the average energy causes the partciles to move even faster. Some partciles at the surface of the liquid have enough energy to overcome the forces of attraction and escape the liquid to form a gas. The liquid becomes to evaporate as the gas is formed.
Eventually, a certain temperature is reached and bubbles of gas start to form inside the liquid. This means the liquid is boiling.
When a gas is cooled, the decrease in average energy causes the particles to move closer together. The force of attraction between the forces becomes significant and the gas condenses into a liquid.
When a liquid is cooled, it freezes to form a solid. Energy is released in each of these changes
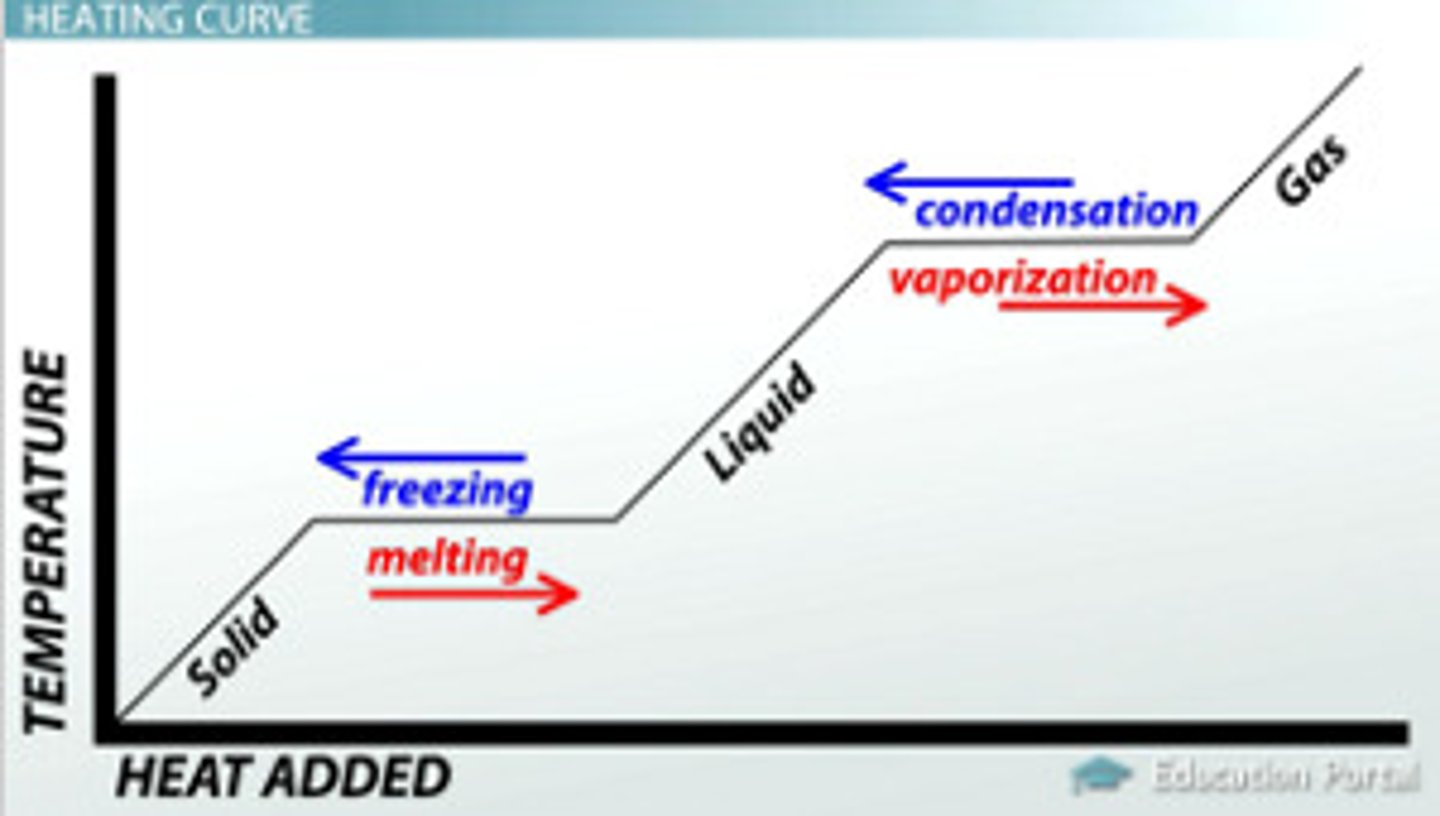
Interpret and explain heating and cooling curves in terms of kinetic particle theory
when a solid is heated, it's temperature increases. When it reached the melting point, the graph shows a horizontal line. This indicated that a change in state is takin place. During a change in state, the temperature remains constant.
When the solid has melted into a liquid, the temperature beings to rise again until is reached the boiling point. At this point, the temperature remains constant because the liquid is changing into a gas.
When the change has taken place, the temperature starts rising again.
The reverse process of condensing and freezing occurs when a subsance is cooled. Energy is given out when the gas condenses to a liquid and the liquid freezez to give a solid.
Describe and explain, in terms of kinetic particle theory, the effects of temperature and pressure on the volume of a gas
When the temperature of a gas increases, the particles move faster, and thus, more frequent and vigorous collisions take place. This causes the particles to 'push' other particles further away. his causes a greater force per area to be exerted, and the pressure increase. This also causes the volume of the gas to increase.
When the temperature increases, the pressure increase causing the volume of the gas to increase as well.
When the temperature decreases, the pressure decreases, causing the volume of the gas to decrease as well.
Describe and explain diffusion in terms of kinetic particle theory
the theory states that all meter is made up of many small particles whichare constantly moving. In a gas, he partciles move randomly past one another, colliding with eachother, and filling any available space. This spreading of gas is called diffusion.
Describe and explain the effect of relative molecular mass on the rate of diffusion of gases
A gas with a smaller relative molecular mass will diffuse more quickly than a gas with a greater relative molecular mass. This is because, the larger the molecule and the higher its molecular weight, the slower its rate of diffusion.
Describe the differences between elements, compounds and mixtures
ELEMENT
A substance made of atoms that all contain the same number of protons and cannot be split into anything simpler
There are 118 elements found in the Periodic Table
COMPOUND
A pure substance made up of two or more elements chemically combined
There is an unlimited number of compounds
Compounds cannot be separated into their elements by physical means
E.g. copper(II) sulfate (CuSO4), calcium carbonate (CaCO3), carbon dioxide (CO2)
MIXTURE
A combination of two or more substances (elements and/or compounds) that are not chemically combined
Mixtures can be separated by physical methods such as filtration or evaporation
E.g. sand and water, oil and water, sulfur powder, and iron filings
Describe the structure of the atom
the atom is a central nucleus containing neutrons and protons surrounded by electrons in shells
State the relative charges and relative masses of a proton, a neutron and an electron
Relaive mass of proton: 1
charge: 1+
relative mass of neutron: 1
charge: 0
relative mass of electron: 1/1840
charge: 1-
Define proton number/ atomic number
the number of protons in the nucleus of an atom
Define isotopes
s different atoms of the same element that have the same number of protons but different numbers of neutrons
do isotopes of the same element have the same or different chemical properties and why?
isotopes of the same element have the same chemical properties because they have the same number of electrons and therefore the same electronic configuration
symbols for atoms
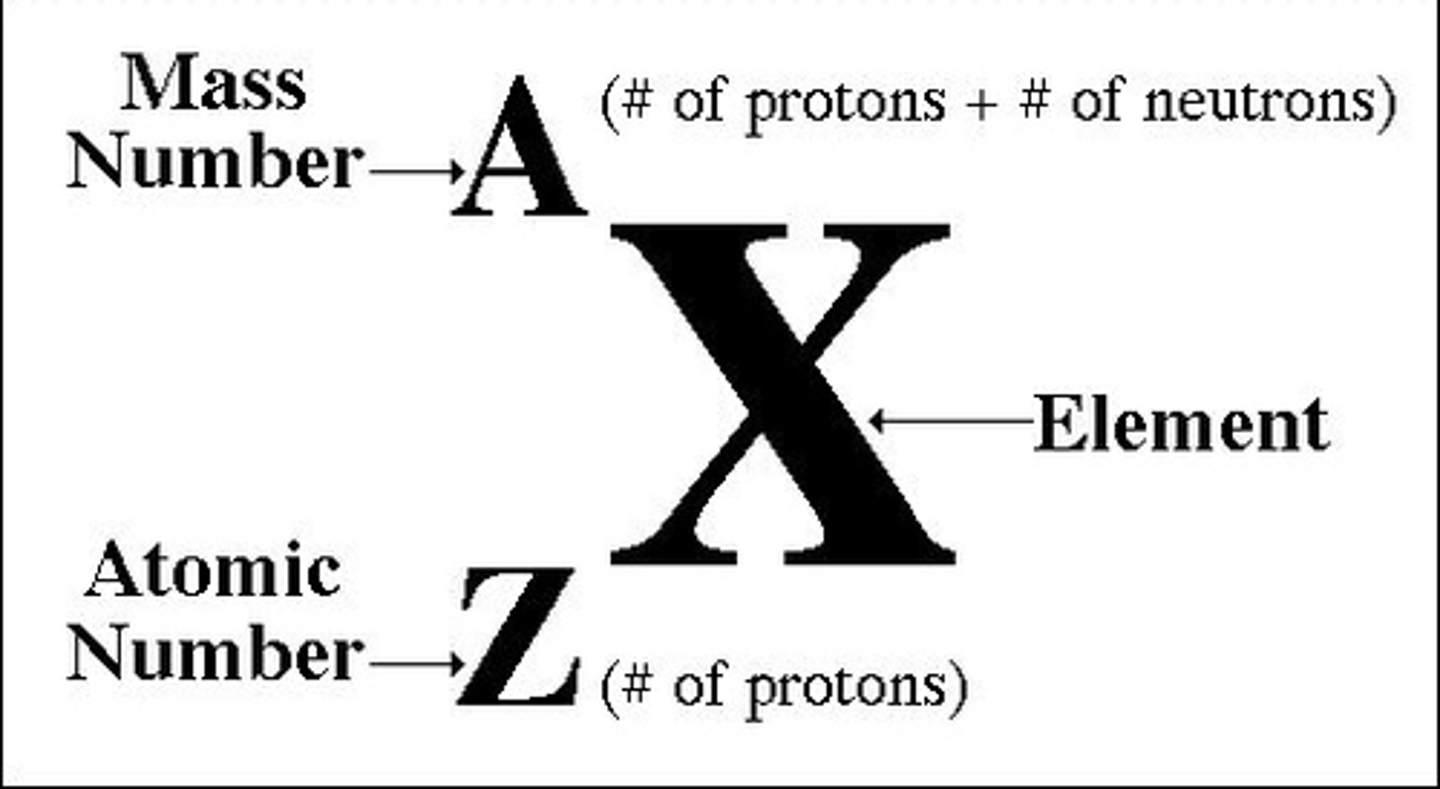
how to determine the electronic configuration of elements and their ions with proton number 1 to 20
- lectrons orbit the nucleus in shells (or energy levels) and each shell has a different amount of energy associated with it
- The further away from the nucleus, the more energy a shell has
- Electrons fill the shell closest to the nucleus and when that becomes full, it fills the next cloest.
- The first shell can hold 2 electrons
- The second shell can hold 8 electrons
- third shell can hold 8 electrons for the first 20 elements.
- The outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
which group has full outer shells
Group VIII noble gases have a full outer shell
what is the number of outer shell electrons is equal to?
the number of outer shell electrons is equal to the group number in Groups I to VII
what is the number of occupied electron shells is equal to?
the number of occupied electron shells is equal to the period number
Calculate the relative atomic mass of an element from the relative masses and abundances of its isotopes
The relative atomic mass of an element can be calculated from the mass number and relative abundances of all the isotopes of a particular element using the following equation:
Ar = (% of isotope 1 x mass of isotope 1) + (% of isotope 2 x mass of isotope 2)/100
Define relative atomic mass
the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of 12C
Is mass number and relative atomic mass the same thing?
- On the Periodic Table provided in your exam you will see that lithium has a relative atomic mass of 7
- Although it seems that this is the same as the mass number, they are not the same thing because the relative atomic mass is a rounded number
- Relative atomic mass takes into account the existence of isotopes when calculating the mass
- Relative atomic mass is an average mass of all the isotopes of that element
- For simplicity relative atomic masses are often shown to the nearest whole number
Describe the formation of positive ions and negative ions
An ion is formed by the loss or gain of electrons
An atom will lose or gain electrons to become more stable by gaining a full outer shell
Metals: all metals can lose electrons to other atoms to become positively charged ions, known as cations
Non-metals: all non-metals can gain electrons from other atoms to become negatively charged ions, known as anions
What is an ionic bond?
A chemical bond that's formed when an atom transfers an electron to another Atom
Describe the giant lattice structure of ionic compounds
The ions have a regular, repeating arrangement called an ionic lattice . The lattice is formed because the ions attract each other and form a regular pattern with oppositely charged ions next to each other.
Describe the formation of ionic bonds between ions of metallic and non-metallic elements
Ionic compounds are formed when metal atoms react with non-metal atoms
The positive and negative ions are held together by strong electrostatic forces of attraction between opposite charges
This force of attraction is known as an ionic bond and they hold ionic compounds together
Describe and explain in terms of structure and bonding the properties of ionic compounds
(a) ionic substances have high melting and boiling points due to the presence of strong electrostatic forces acting between the oppositely charged ions
(b) Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a charge
They are poor conductors in the solid state as the ions are in fixed positions within the lattice and are unable to move
What is a covalent bond?
a covalent bond is formed when a pair of electrons is shared between two non-metal atoms leading to noble gas electronic configurations
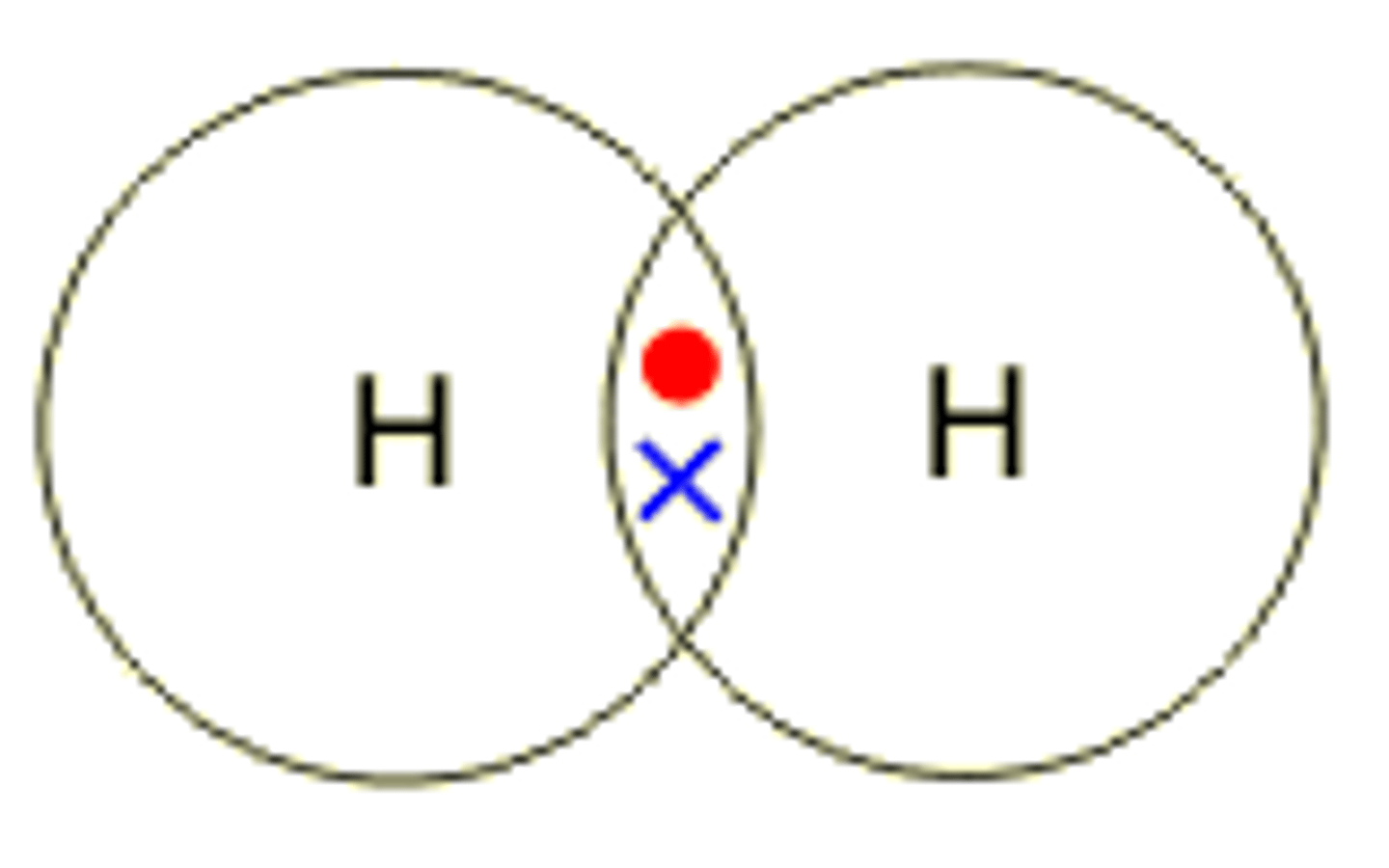
Describe the formation of covalent bonds in H2
hydrogen
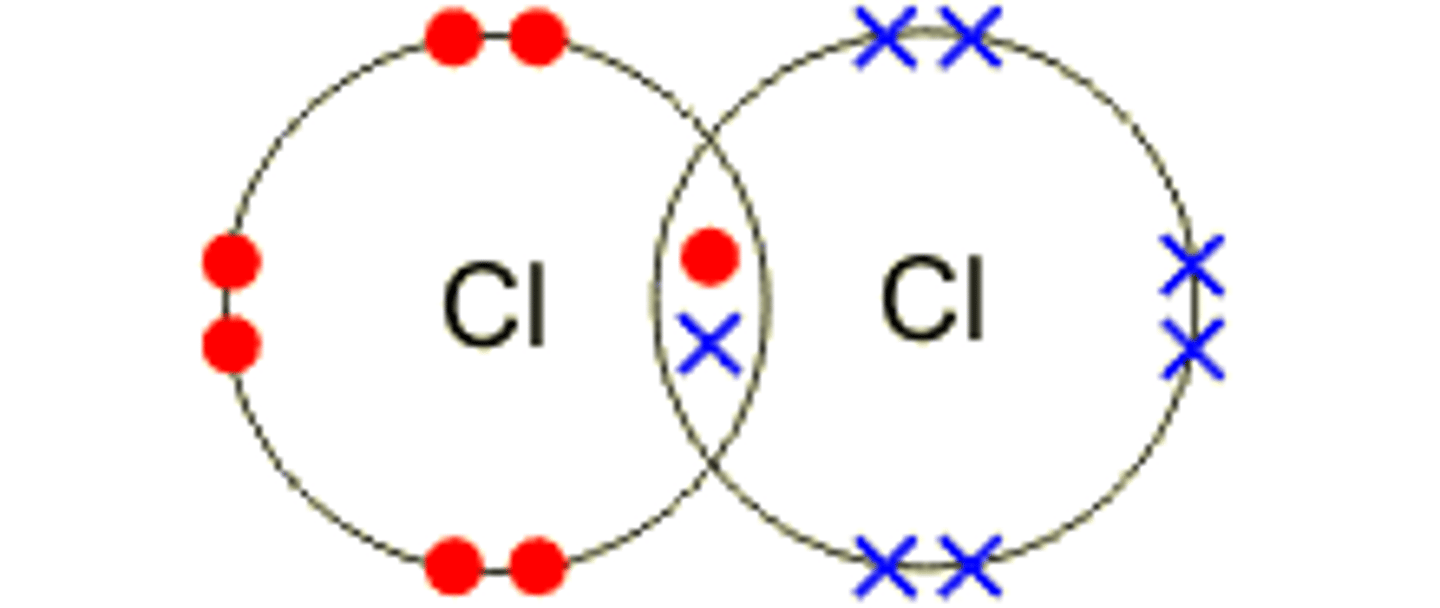
Describe the formation of covalent bonds in Cl 2
chloride
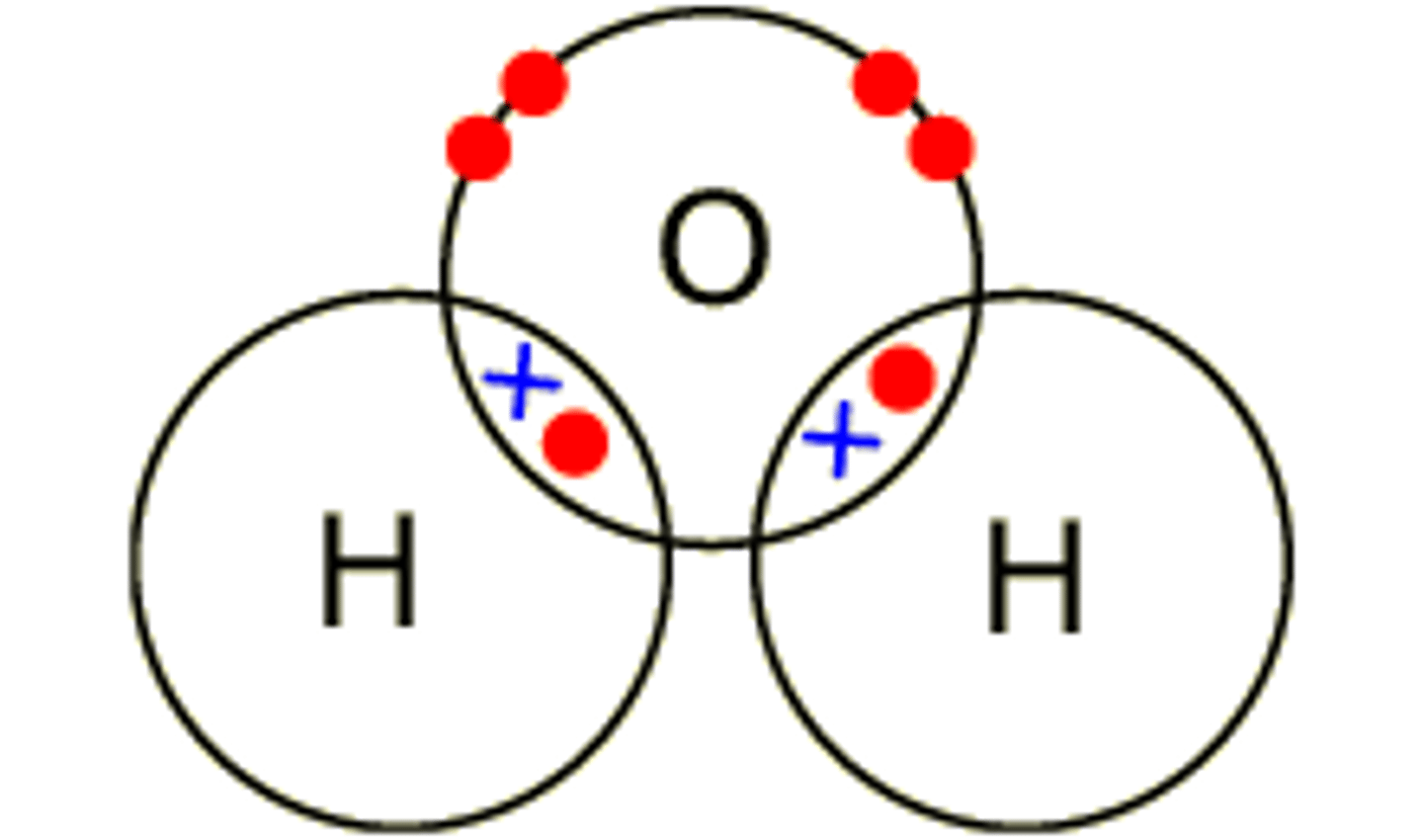
Describe the formation of covalent bonds in H2O
water
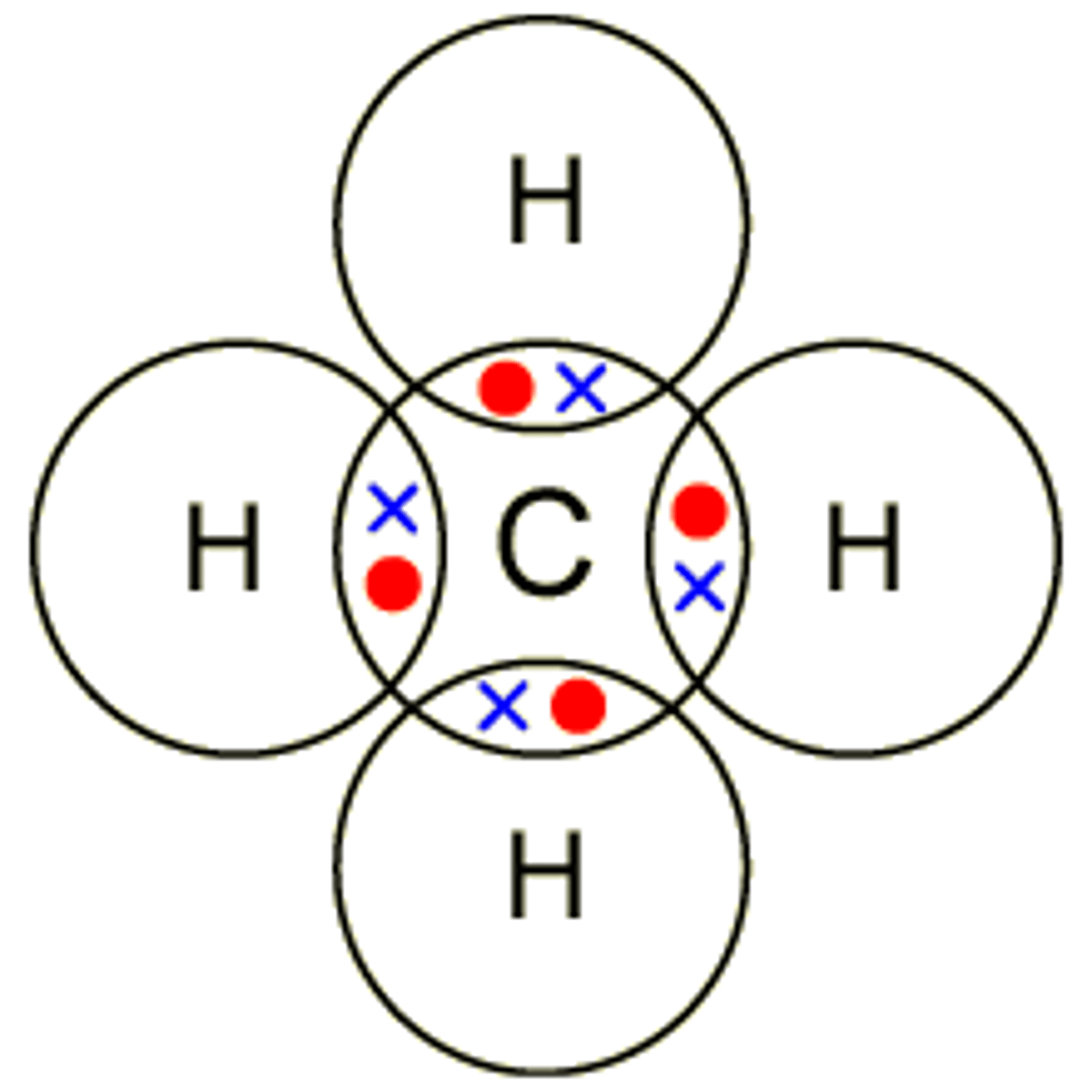
Describe the formation of covalent bonds in CH4
methane
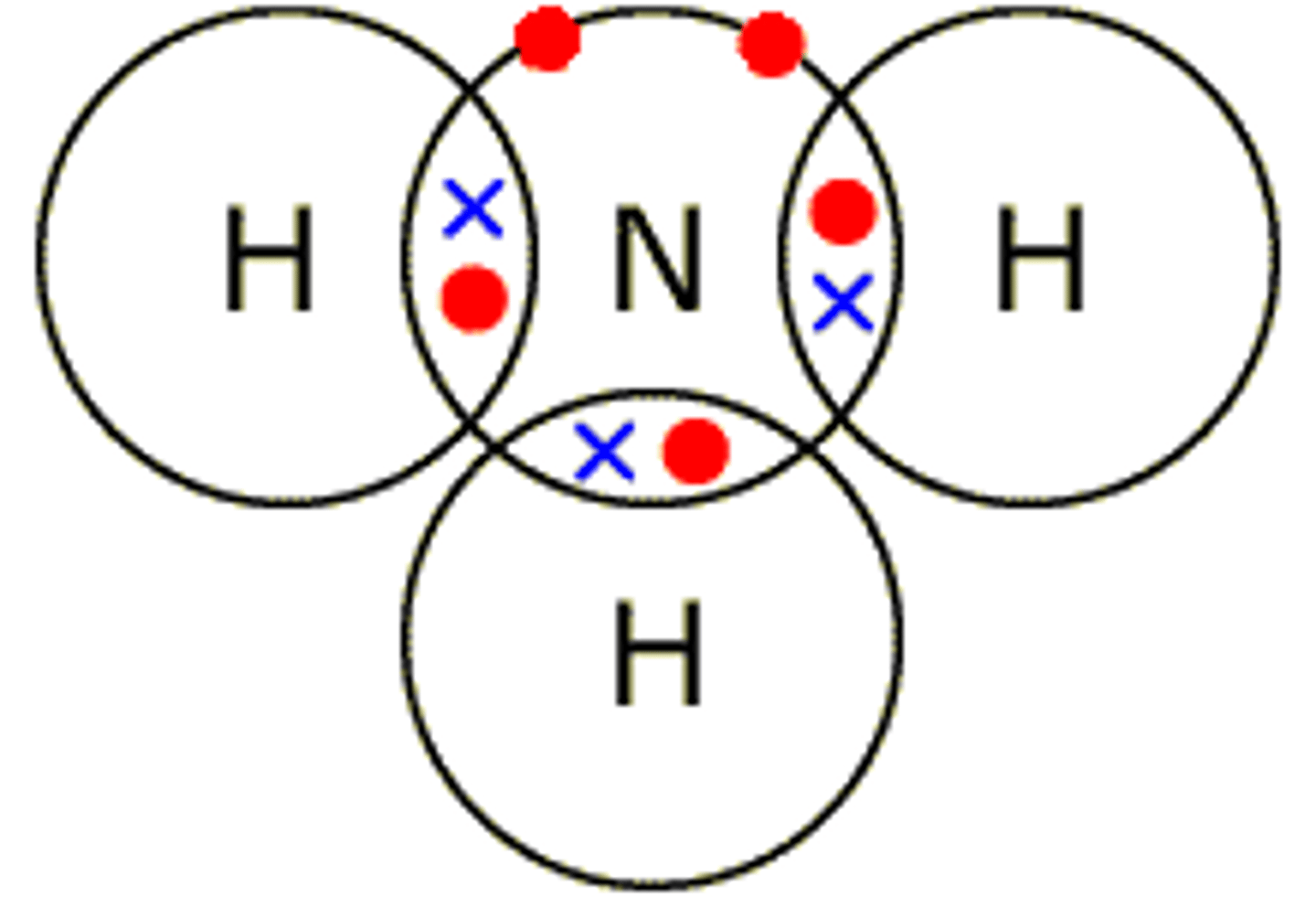
Describe the formation of covalent bonds in NH3
ammonia
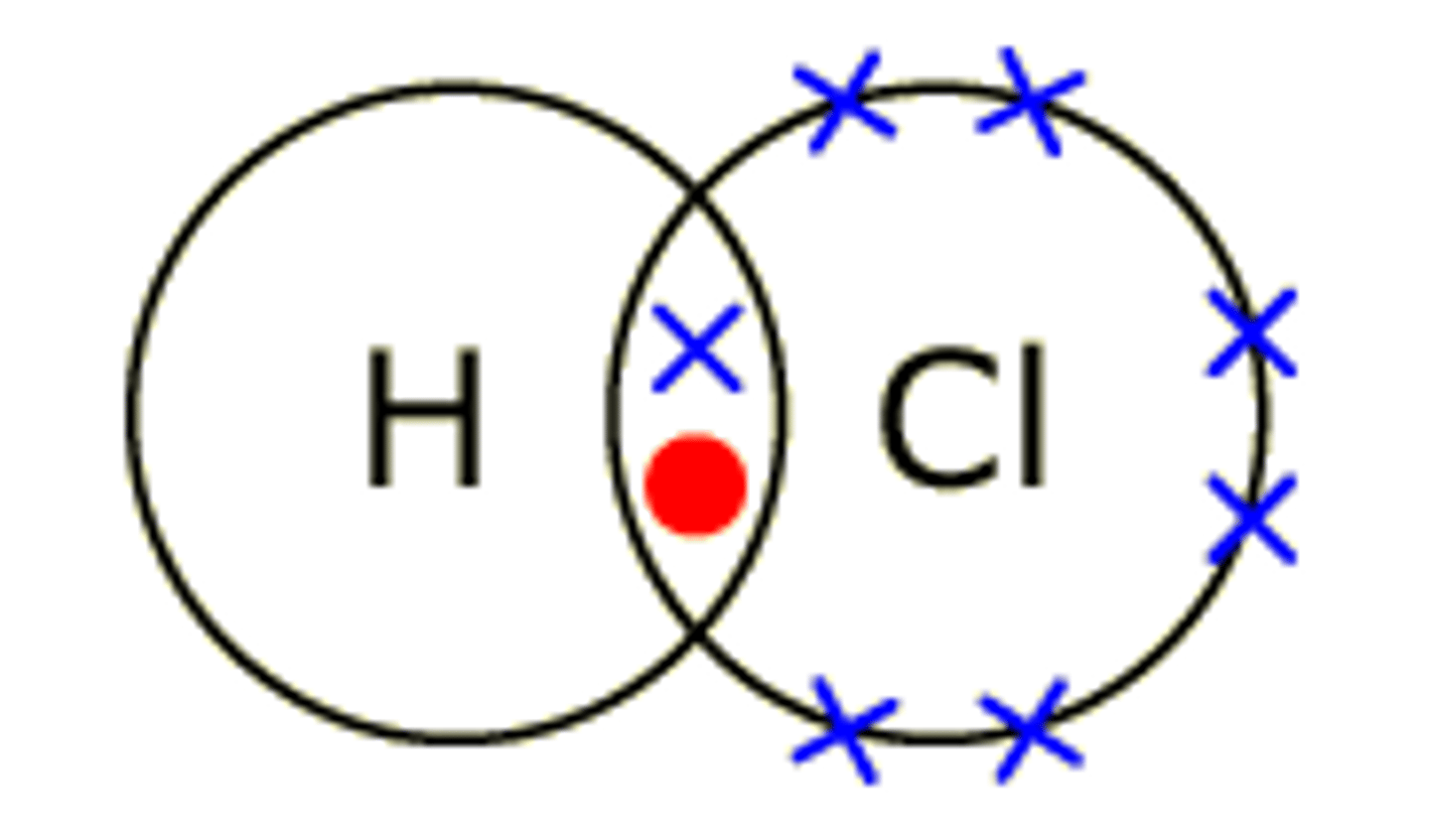
Describe the formation of covalent bonds in HCl
Hydrogen chloride
Describe the formation of covalent bonds in C2H4
Ethylene
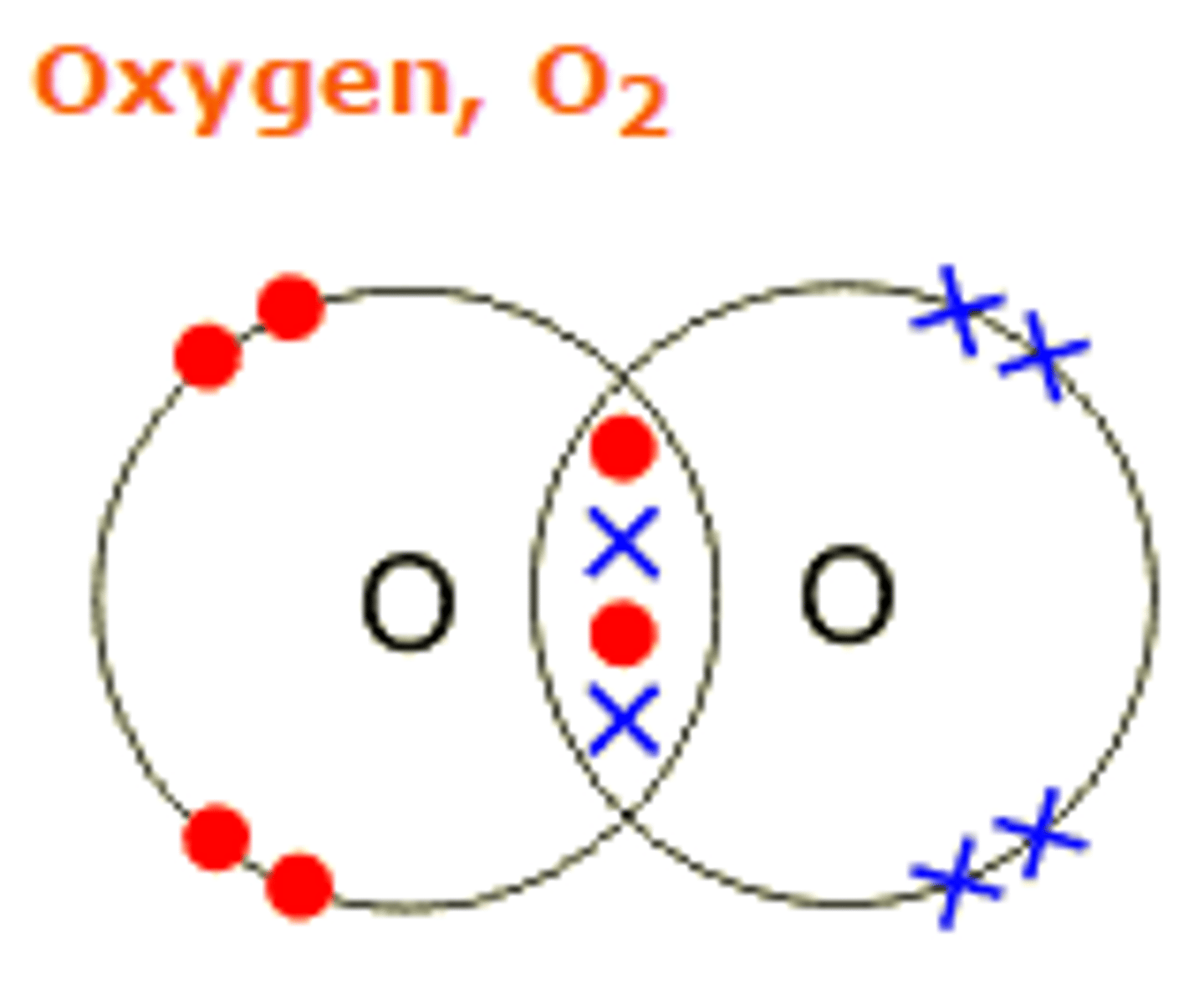
Describe the formation of covalent bonds in O2
oxygen
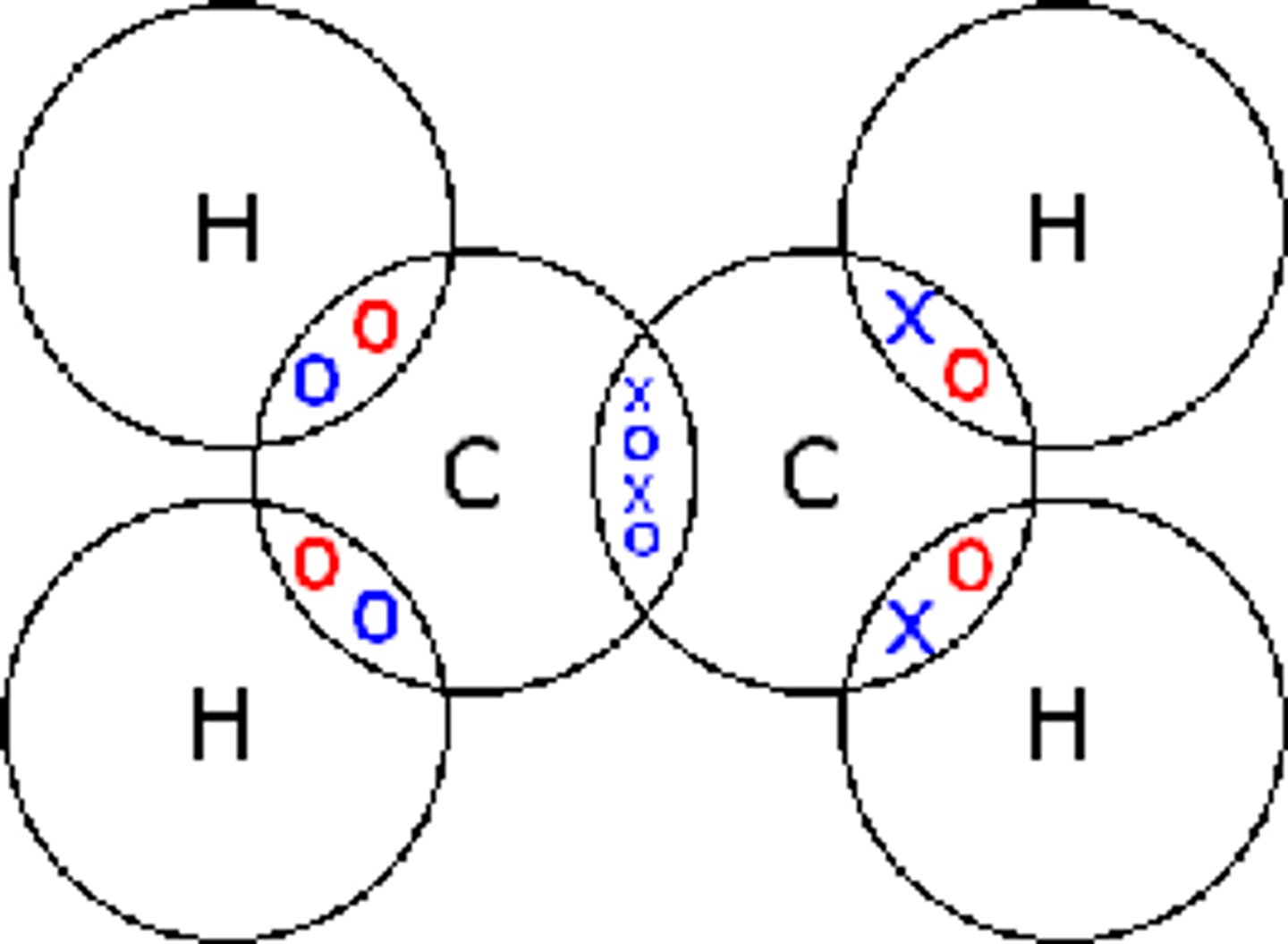
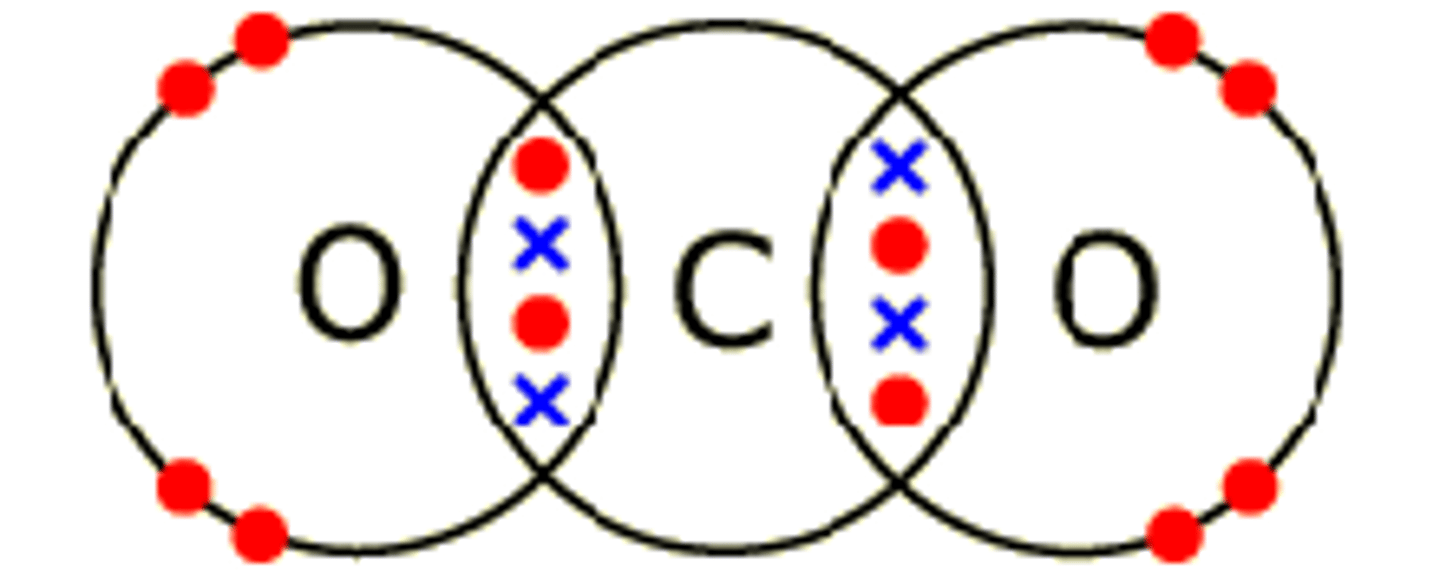
Describe the formation of covalent bonds in CO2
carbon dioxide
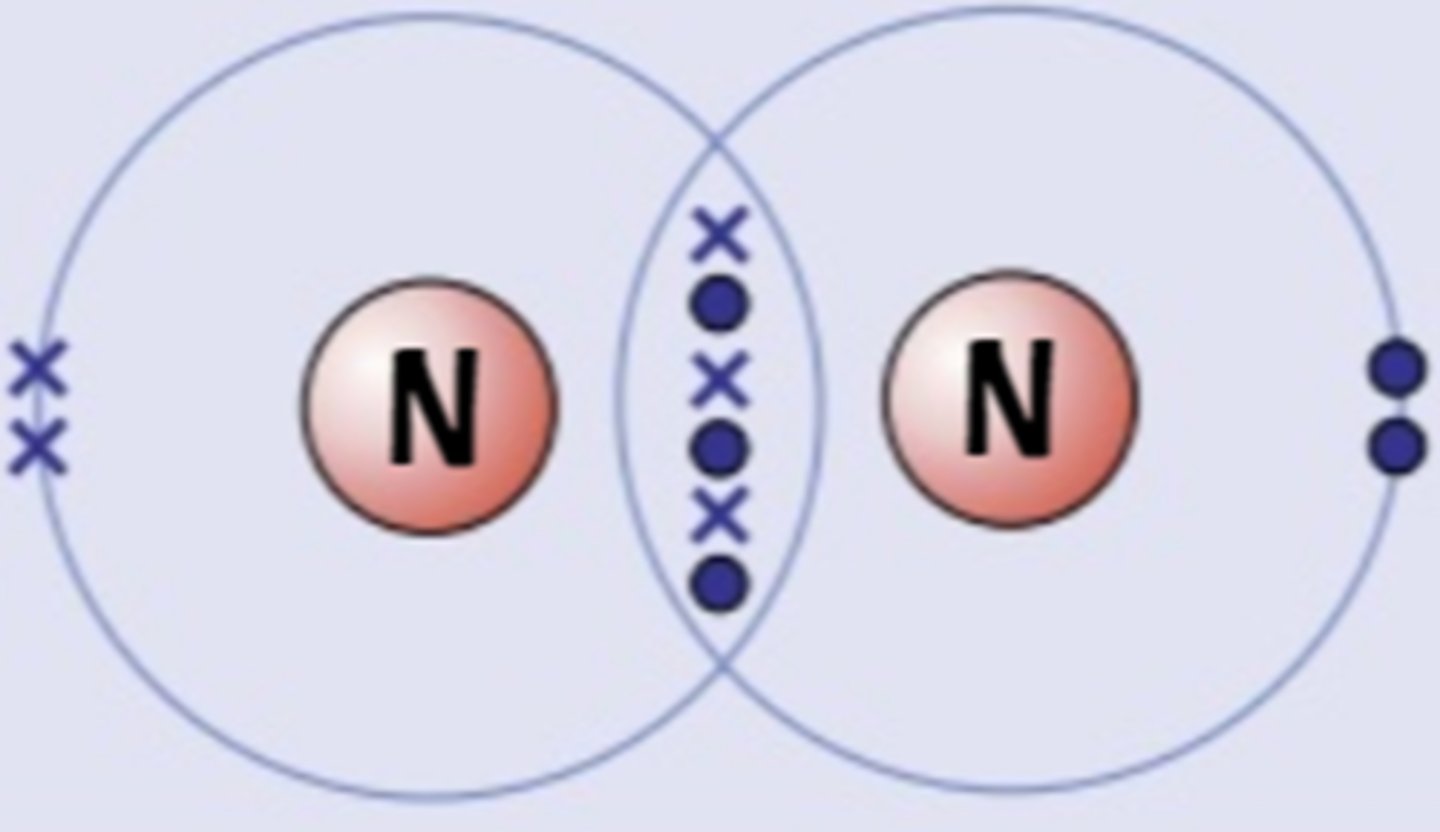
Describe the formation of covalent bonds in N2
nitrogen
Describe the formation of covalent bonds in CH3OH
Methanol - carbon is attached to 3 H and 1 OH with 4 covalent bonds
Describe and explain in terms of structure and bonding the properties of simple molecular compounds
(a) They have low melting and boiling points as there are only weak intermolecular forces acting between the molecules
(b) Molecular compounds are poor conductors of electricity as there are no free ions or electrons to carry the charge.
Describe the giant covalent structures of graphite
Each carbon atom in graphite is bonded to three others forming layers of hexagons, leaving one free electron per carbon atom which becomes delocalised
The covalent bonds within the layers are very strong, but the layers are attracted to each other by weak intermolecular forces
Describe the giant covalent structures of diamond
In diamond, each carbon atom bonds with four other carbons, forming a tetrahedron
All the covalent bonds are identical, very strong and there are no intermolecular forces
Relate the structures and bonding of graphite and diamond to their uses
(a) graphite - Conducts electricity; has a very high melting point; Is soft and slippery; less dense than diamond
Graphite is used in pencils and as an industrial lubricant, in engines and in locks
It is also used to make non-reactive electrodes for electrolysis
(b) diamond - It does not conduct electricity; has a very high melting point; is extremely hard and dense
Diamond is used in jewelry due to its sparkly appearance and as a cutting tool as it is such a hard material
Describe the giant covalent structures of silicon(IV) oxide, SiO2
Silicon(IV) oxide, SiO2, is a macromolecular compound which occurs naturally as sand and quartz
Each oxygen atom forms covalent bonds with 2 silicon atoms and each silicon atom in turn forms covalent bonds with 4 oxygen atoms, forming a tetragedron
Describe the similarity in properties between diamond and silicon(IV) oxide, related to their structures
1. SiO2 has lots of very strong covalent bonds and no intermolecular forces so it has similar properties to diamond
2. It is very hard, has a very high boiling point, is insoluble in water, and does not conduct electricity
3. SiO2 is cheap since it is available naturally and is used to make sandpaper and to line the inside of furnaces
Describe metallic bonding
Within the metallic lattice, the atoms lose the electrons from their outer shell and become positively charged ions
The outer electrons no longer belong to a particular metal atom and are said to be delocalized
They move freely between the positive metal ions like a 'sea of electrons'
Metallic bonds are strong and are a result of the attraction between the positive metal ions and the negatively charged delocalized electrons
Explain in terms of structure and bonding the properties of metals
1. Metals have high melting and boiling points
There are many strong metallic bonds in giant metallic structures between the positive metal ion and delocalized electrons
2. Metals conduct electricity
There are free electrons available to move through the structure and carry a charge
3. Metals are malleable and ductile
Layers of positive ions can slide over one another and take up different positions
structural formula
The structural formula tells you the way in which the atoms in a particular molecule are bondedThis can be done by either a diagram (displayed formula) or written (simplified structural formula)
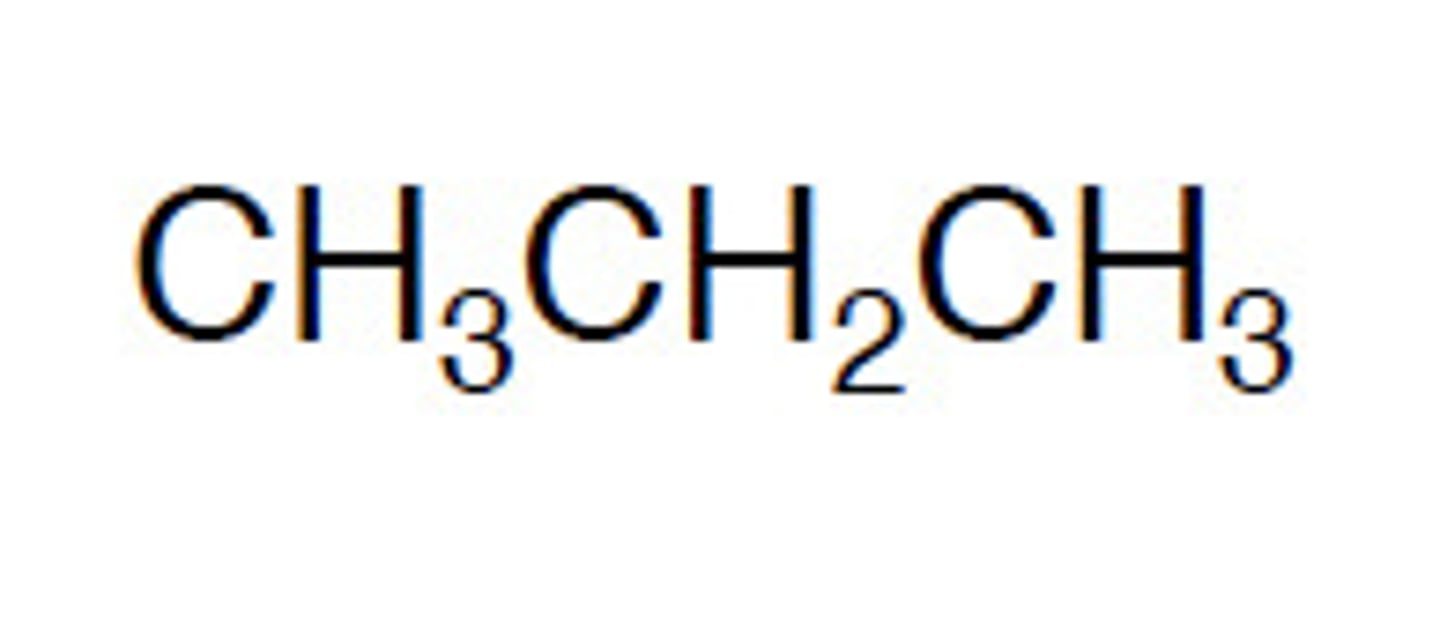
molecular formula
The molecular formula is the formula that shows the number and type of each atom in a molecule
it tells you the actual number of atoms of each element in one molecule of the compound or element
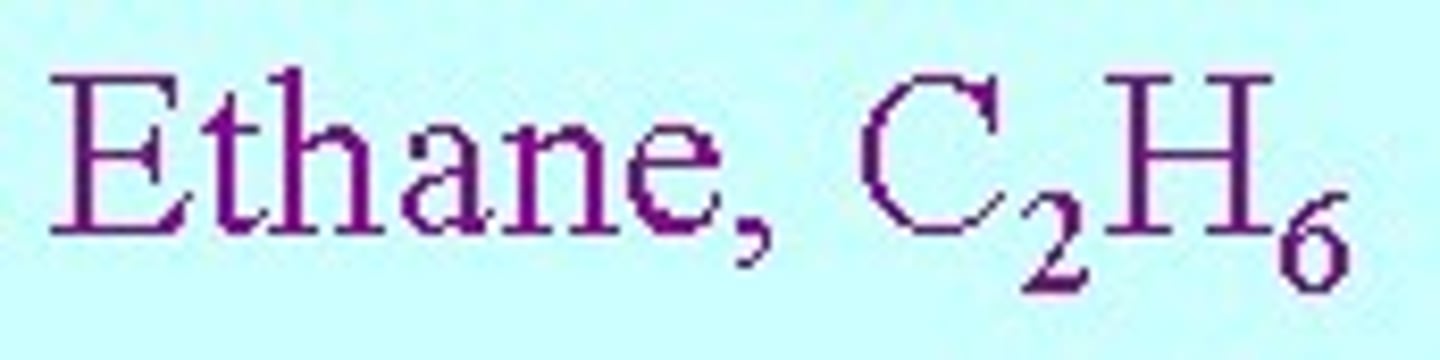
empirical formula
empirical formula of a compound is the simplest whole number ratio of the different atoms or ions in a compound
how to deduce formulae by combining power/valency/relative no. of atoms or ions present
group I, valency 1
group II, valency 2
group III, valency 3
group IV, valency 4
group V, valency 3
group VI, valency 2
group VII, valency 1
group VIII, valency 0
step 1
write donw symbold of each element and write their combining powers
Al/3 and S/2
step 2
the formula is then calculated by cross mutiplying each atom with the number opposite
Al with 2 and S with 3
step 3
the formula for aluminium sulfide is Al2S3
The formula of an ionic compound is always an empirical formula. true or false?
true
charges of elements from different groups of periodic table
Group I elements form ions with a 1+ charge
Group II elements form ions with a 2+ charge
Group III elements form ions with a 3+ charge
Group V elements form ions with a 3- charge
Group VI elements form ions with a 2- charge
Group VII elements form ions with a 1- charge
Deducing Formulae of Ionic Compounds by the elements charge
What is the formula of sodium bromide?
Answer:
symbol: Na and Br
ion charge: 1+ and 1_
balance the number of ions: 1 sodium ion is needed for each bromide ion
ratio of ions: 1:1
formula: NaBr
how to write a word equation
These show the reactants and products of a chemical reaction using their full chemical names
The arrow (which is spoken as "goes to" or "produces") implies the conversion of reactants into products
Reaction conditions or the name of a catalyst can be written above the arrow
reactant 1 + reactatant 2 --> product
how to name a compound?
1- For compounds consisting of 2 atoms:
1. If one is a metal and the other a non-metal, then the name of the metal atom comes first and the ending of the second atom is replaced by adding -ide.
2. If both atoms are non-metals and one of those is hydrogen, then hydrogen comes first
3. For other combinations of non-metals as a general rule, the element that has a lower group number comes first in the name
- For compounds that contain certain groups of atoms:
4. There are common groups of atoms which occur regularly in chemistry
When these ions form a compound with a metal atom, the name of the metal comes first
What are the state symbols?
State symbols are written after each formula in chemical equations to show which physical state each substance is in
i.e. (s), (l), (g), (aq)
how to identify a physical state of elements and compounds?
Generally, unless they are in a solution:
1. Metal compounds will always be solid, although there are a few exceptions
2. Ionic compounds will usually be solids
3. Non-metal compounds could be solids, liquids or gases, so it depends on chemical structure
4. Precipitates formed in solution count as solids
Balancing ionic equations
Q. Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide.
Answer:
Step 1: Write out the full balanced equation:
2KI (aq) + Cl2 (aq) → 2KCl (aq) + I2 (aq)
Step 2: Identify the ionic substances and write down the ions separately
2K+ (aq) + 2I- (aq) + Cl2 (aq) → 2K+ (aq) + 2Cl- (aq) + I2 (aq)
Step 3: Rewrite the equation eliminating the ions which appear on both sides of the equation (spectator ions ) which in this case are the K+ ions:
2I- (aq) + Cl2 (aq) → 2Cl- (aq) + I2 (aq)
Describe relative atomic mass, Ar
the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of 12C
Define relative molecular mass, Mr
the sum of the relative atomic masses. (Relative formula mass, Mr, will be used for ionic compounds)
mole
the mole, mol, is the unit of amount of substance and that one mole contains 6.02×1023 particles, e.g. atoms, ions, molecules; this number is the Avogadro constant
formula for amount of substance (mol)
amount of substance (mol) = mass (g)/molar mass (g /mol)
mnemonic: Mr. Moles = mass
molar mass
The mass of 1 mole of a substance is known as the molar mass
For an element, it is the same as the relative atomic mass written in grams
For a compound it is the same as the relative formula mass or relative molecular mass in grams
Molar gas volume
At room temperature and pressure, the volume occupied by one mole of any gas was found to be 24 dm3 or 24,000 cm3
This is known as the molar gas volume at RTP
molar gas volume formula
amount of gas (mol) = volume (dm3)/24 dm3 mol-1
calculating mass of reactants and products
Q. Calculate the mass of magnesium oxide that can be made by completely burning 6.0 g of magnesium in oxygen in the following reaction:
2Mg (s) + O2 (g) ⟶ 2 MgO (s)
Relative formula masses (Mr): Mg = 24; MgO = 40
Ans:
STEP 1
calculate the moles of magnesium used in the reaction
moles = 6/24 = 0.25
STEP 2
find the ratio of Mg to MgO using the molar ratio from the balanced equation
2Mg:2MgO
ratio is thus 1:1
so, 0.25 moles Mg produces 0.25 moles of MgO
STEP 3
calculate the mass of MgO
mass = 0.25 x 40 = 10kg
limiting reactant
- The reactant that is used up first in a reactionis the limiting reactant, as it limits the duration and hence the amount of product that a reaction can produce
- The amount of product is therefore directly proportional to the amount of the limiting reactant added at the beginning of a reaction
- The limiting reactant is the reactant which is not present in excess in a reaction
how to determine which reactant is the limiting reactant ina reaction
When performing reacting mass calculations, the limiting reactant is always the number that should be used as it indicates the maximum possible amount of product
The steps are:
Write the balanced equation for the reaction
Calculate the moles of each reactant
Compare the moles & deduce the limiting reactant
what is a solue, solvent and a solution?
A solid substance that dissolves in a liquid is called a solute, the liquid is called a solvent and the two when mixed together form a solution
What is concentration?
Concentration simply refers to the amount of solute there is in a specific volume of the solvent
concentration formula
concentration (g/dm3) = mass of solute (g)/ volume of solution (dm3)
OR
concentration (mol/dm3) = number of moles of solute (mol)/volume of soluion (dm3)
conversion between dm3 and cm 3
1 decimetre cubed (dm3) = 1000 cm3
1 decimetre cubed (dm3) is the same as 1 litre
conversion betweeb g/dm3 to mol/dm3
To go from g/dm3 to mol/dm3 Divide by the molar mass in grams
To go from mol/dm3 to g/dm3Multiply by the molar mass in grams
Titration calculation steps
Q. A solution of 25.0 cm3 of hydrochloric acid was titrated against a solution of 0.100 mol/dm3 NaOH and 12.1 cm3 were required for complete reaction. Determine the concentration of the acid.
Ans:
Step 1: Write the equation for the reaction:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Step 2: Calculate the number of moles of the NaOH
Moles = (volume ÷ 1000) x concentration
Moles of NaOH = 0.012 dm3 x 0.100 mol/dm3 = 1.21 x 10-3 mol
Step 3: Deduce the number of moles of the acid
Since the acid reacts in a 1:1 ratio with the alkali, the number of moles of HCl is also 1.21 x 10-3 mol
This is present in 25.0 cm3 of the solution (25.0 cm3 = 0.025 dm3)
Step 4: Find the concentration of the acid
Concentration = moles ÷ volume (dm3)
Concentration of HCl =1.21 x 10-3 mol ÷ 0.025 dm3 = 0.0484 mol/dm3
Molecular formula
Molecular formula gives the actual numbers of atoms of each element present in the formula of the compound
steps to calculate the molecular formula
Step 1: Find the relative formula mass of the empirical formula
Step 2: Use the following equation:
relative formula mass of molecular frmula/relative forula mass of empirical formula
Step 3: Multiply the number of each element present in the empirical formula by the number from step 2 to find the molecular formula
state some reason about why you never get 100% yield in a chemical process
1. Some reactants may be left behind in the equipment
2. The reaction may be reversible and in these reactions a high yield is never possible as the products are continually turning back into the reactants
3. Some products may also be lost during separation and purification stages
4. There may be side reactions occurring where a substance reacts with a gas in the air or an impurity in one of the reactants
5. Products can also be lost during transfer from one container to another
diffrentiate between actual yield, theoretical yield, and percentage yield
The actual yield is the recorded amount of product obtained
The theoretical yield is the amount of product that would be obtained under perfect practical and chemical conditions
The percentage yield compares the actual yield to the theoretical yield
formula for percentage yield
percentage yield = actual yield/theoretical yield x 100
how to find the percentage by mass of an element within a compoun
% of an element = total mass of the element in the compound/relative formula mass of the compound x 100
formula for percentage purity
% purity = mas of pure substance/toal mass of substance x 100
Define electrolysis
the decomposition of an ionic compound, when molten or in aqueous solution, by the passage of an electric current
Identify in simple electrolytic cells:
(a) the anode
Anode is the positive electrode of an electrolysis cell
Identify in simple electrolytic cells
(b) the cathode
Cathode is the negative electrode of an electrolysis cell
Identify in simple electrolytic cells:
(c) the electrolyte
Electrolyte is the ionic compound in a molten or dissolved solution that conducts the electricity
Describe the transfer of charge during electrolysis to include
- The power supply provides the cathode with a supply of electrons, causing it to become negatively charged
- Positive ions (cations) in the electrolyte move towards the cathode where they gain electrons
- Negative ions (anions) in the electrolyte move towards the anode where they lose electrons
- The electrons move from the anode back towards the power supply
- So, in a complete circuit: Electrons are the charge carriers in the external circuit
Ions are the charge carriers in the electrolyte
Identify the products formed at the electrodes and describe the observations made during the electrolysis of:
(a) molten lead(II) bromide
using inert electrodes made of platinum or carbon/ graphite
1. Add lead(II) bromide into a beaker and heat it so it will turn molten, allowing ions to be free to move and conduct an electric charge
2. Add two graphite rods as the electrodes and connect this to a power pack or battery
3. Turn on the power pack or battery and allow electrolysis to take place
4. Negative bromide ions move to the positive electrode (anode) and each loses one electron to form bromine molecules. There is bubbling at the anode as brown bromine gas is given off
5. Positive lead ions move to the negative electrode (cathode) and gain electrons to form a grey lead metal which deposits on the surface of the electrode
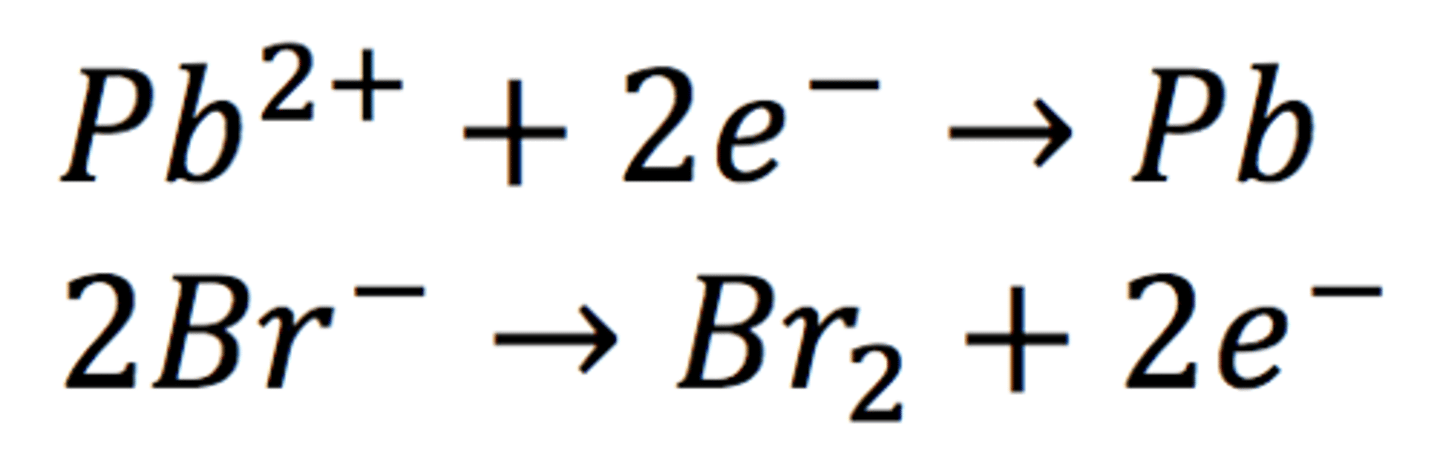
Identify the products formed at the electrodes and describe the observations made during the electrolysis of:
(b) concentrated aqueous sodium chloride
using inert electrodes made of platinum or carbon/ graphite
1. Brine is a concentrated solution of aqueous sodium chloride
2. It can be electrolysed using inert electrodes made from platinum or carbon/graphite
3. When electrolysed, it produces bubbles of gas at both electrodes as chlorine and hydrogen are produced, leaving behind sodium hydroxide solution
Product at the Negative Electrode:
The H+ ions are discharged at the cathode as they are less reactive than sodium ions
The H+ ions gain electrons to form hydrogen gas
Product at the Positive Electrode:
The Cl- ions are discharged at the anode
They lose electrons and chlorine gas forms
The Na+ and OH- ions remain behind and form the NaOH solution
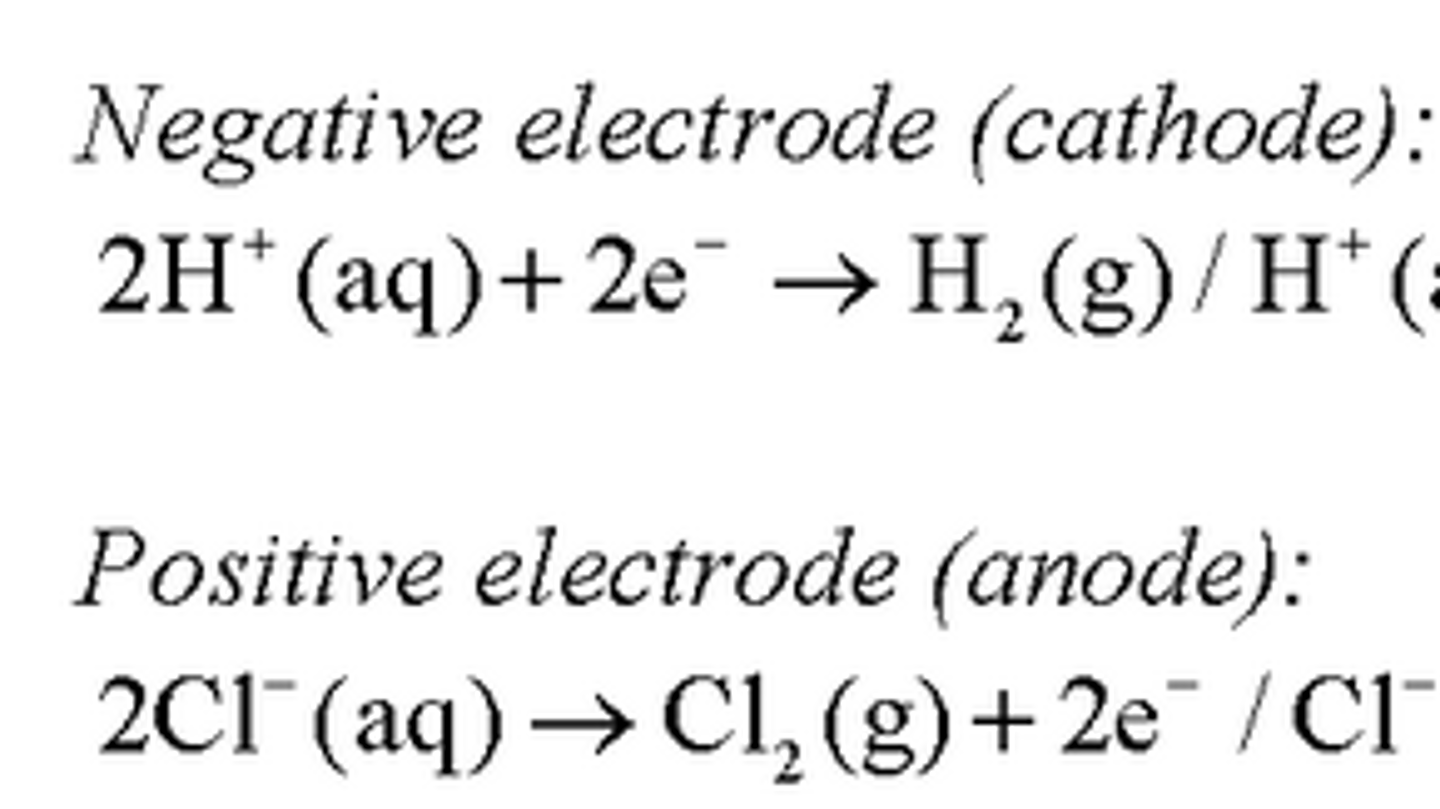
Identify the products formed at the electrodes and describe the observations made during the electrolysis of:
(c) dilute sulfuric acid
using inert electrodes made of platinum or carbon/ graphite
1. Dilute sulfuric acid can be electrolysed using inert electrodes made from platinum or carbon/graphite
2. Bubbles of gas are seen at both electrodes
Product at the Negative Electrode
H+ ions are attracted to the cathode, gain electrons and form hydrogen gas
Product at the Positive Electrode
OH- ions are attracted to the anode, lose electrons and form oxygen gas and water
Identify the products formed at the electrodes and describe the observations made during the electrolysis of aqueous copper(II) sulfate using inert carbon/ graphite electrodes and when using copper electrodes
USING GRAPHITE ELECTRODES:
Product at the Cathode:
Copper is less reactive than hydrogen
Copper ions are discharged at the cathode, gain electrons and are reduced to form copper metal
Cu2+ + 2e- → Cu
Product at the Anode:
OH- ions lose electrons more readily than SO42
OH- lose electrons and are oxidised to form oxygen gas
4OH- ⟶ O2 + 2H2O + 4e-
USING COPPER ELECTRODES:
Observations at the anode and cathode:
- copper atoms are oxidised at the anode and form ions while copper ions are reduced at the cathode, forming copper atoms
- The gain in mass by the negative electrode is the same as the loss in mass by the positive electrode
- Therefore the copper deposited on the negative electrode must be the same copper ions that are lost from the positive electrode
- That implies that the concentration of the Cu2+ ions in the solution remains constant
what type of subsances are formed at the cathode and anode?
metals or hydrogen are formed at the cathode and that non-metals (other than hydrogen) are formed at the anode
Predict the identity of the products at each electrode for the electrolysis of a binary compound in the molten state
To predict the products made at each electrode, first identify the ions
The positive ion will migrate towards the cathode and the negative ion will migrate towards the anode
Therefore, the cathode product will always be the metal, and the product formed at the anode will always be the non-metal
Predict the identity of the products at each electrode for the electrolysis of a halide compound in dilute or concentrated aqueous solution
POSITIVE ELECTRODE (ANODE)
1. If halide ions and OH- are present then the halide ion is discharged at the anode and forms a halogen).
2. If no halide ions are present, then OH- is discharged at the anode and forms oxygen gas
3. The concentration of the solution also affects which ion is discharged:
a. If a concentrated halide solution is being electrolysed, the halogen forms at the anode
b. If a dilute halide solution is being electrolysed, oxygen is formed
NEGATIVE ELECTRODE (CATHODE)
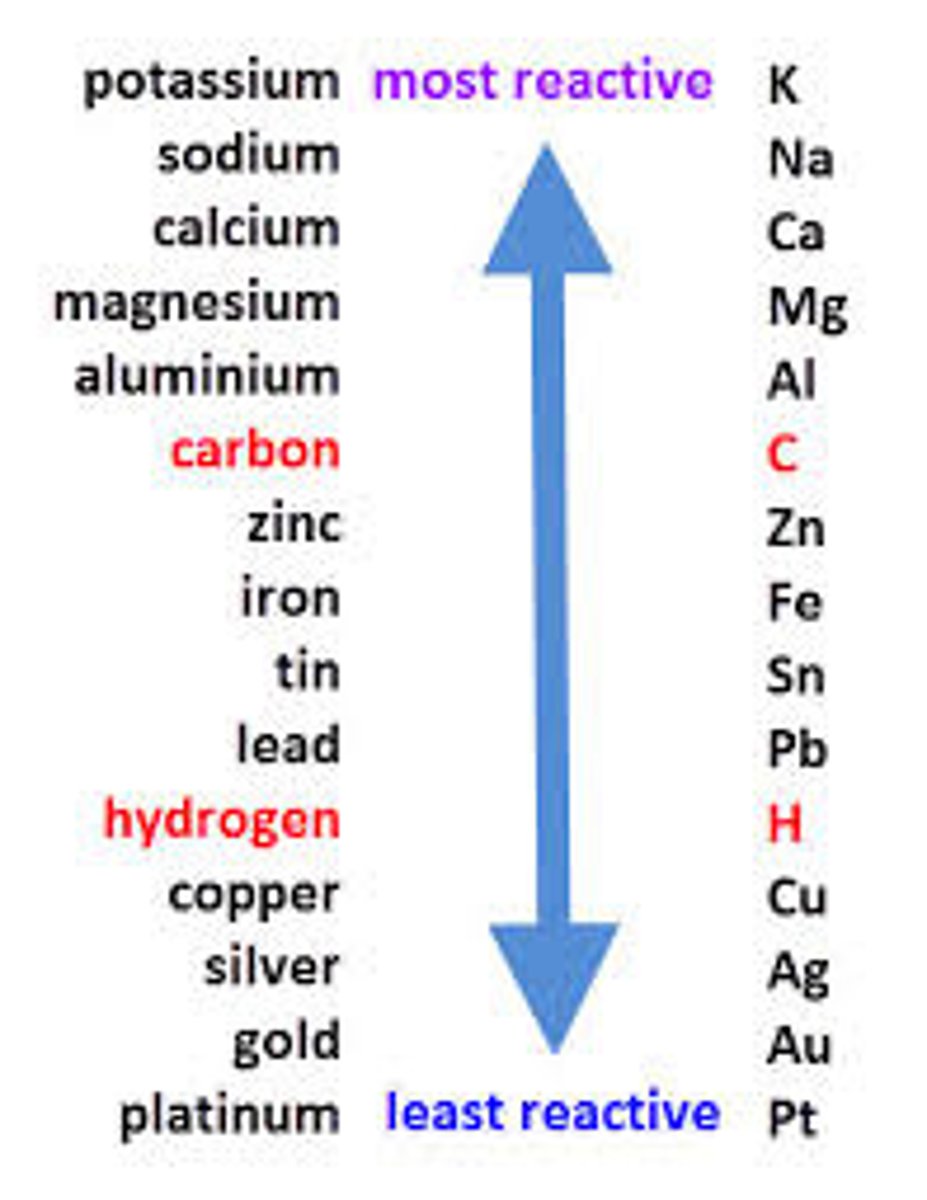
1. If the metal is above hydrogen in the reactivity series, then hydrogen will be produced.
This is because the ions of the more reactive metal will remain in the solution, causing the ions of the least reactive metal to be discharged
2. If the positive ions from the ionic compound are less reactive than hydrogen, the metal is produced
Why are metal objects electroplated?
to improve their appearance and resistance to corrosion
Describe how metals are electroplated
1. The anode is made from the pure metal you want to coat your object with
2. The cathode is the object to be electroplated
3. The electrolyte is an aqueous solution of a soluble salt of the pure metal at the anode
At the anode: metal1 (the coating) atoms lose electrons to form ions in solution
At the cathode: metal1 ions gain electrons to form metal1 atoms which deposit on the strip of the metal2 (to be coated), coating it with a layer of the metal1
hydrogen-oxygen fuels
a hydrogen-oxygen fuel cell uses hydrogen and oxygen to produce electricity with water as the only chemical product
Describe the advantages and disadvantages of using hydrogen-oxygen fuel cells in comparison with gasoline /petrol engines in vehicles
(comparing hydrogen-oxygen fuels to petrol or diesel)
Advantages
1. do not produce any pollution: the only product is water whereas petrol engines produce carbon dioxide, and oxides of nitrogen
2. release more energy per kilogram
3. No power is lost in transmission as there are no moving parts, unlike an internal combustion engine
4. less noise pollution
Disadvantages
1. Materials used in producing fuel cells are expensive
2. Hydrogen is more difficult and expensive to store as it is very flammable and easily explodes when under pressure
3. Fuel cells are affected by low temperatures, becoming less efficient
4. There are only a small number of hydrogen filling stations across the country
5. Hydrogen is often obtained by methods that involve the combustion of fossil fuels, therefore releasing carbon dioxide and other pollutants into the atmosphere
Determining what gas is produced at the electrodes
If the gas produced at the anode relights a glowing splint dipped into a sample of the gas then the gas is oxygen
If the gas produced at the anode bleaches damp litmus paper then the gas is chlorine
If the gas produced at the cathode burns with a 'pop' when a sample is lit with a lighted splint then the gas is hydrogen