6.3- Manipulating the Genome
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
Sanger Sequencing Method
DNA mixed with primers, DNA polymerase, normal nucleotide bases and terminator bases
DNA split into single strands and copied multiple times
DNA polymerase adds nucleotides to single stranded template
when terminator base added to DNA, synthesis stops→ tagged with fluorescent colour
produces DNA fragments of all possible lengths
Fragments separated by length→ gel electrophoresis
laser detects fluorescent colours of bases to determine their sequence order
advancements in sequencing
parallel sequencing→ allows simultaneous sequencing of millions of DNA fragments
Exponentially increased speed→ bacterial genome can be sequenced in less than 24 hours
reduced costs→ can sequence genomes of more organisms
gel electrophoresis
separates DNA fragments by length
Phosphate groups negatively charged→ attracted to anode
shorter fragments move at faster rates→ fragments move different distances over time
what happens during electrophoresis
voltage applied across the gel
fragments of of DNA OR RNA move towards anode
fragments travel at different speeds and therefore separate by size
analysing results from gel electrophoresis
stain applied to DNA/RNA to reveal bands of fragments
Migration distances assessed to approximate sizes of fragments
genetic fingerprinting
also known as DNA profiling
used to identify DNA patterns in individuals
helps identify individuals in forensics or family relationships
relies on the fact that nearly every person’s DNA sequence is distinct
variable number tandem repeats
unique, non-coding, repetitive DNA segments
variation in sequence and length of VNTRs= distinct DNA sequences
key features of VNTRs
present across genomes of most eukaryotes
not involved in protein coding
very variable in sequence and length among individuals
length and location inheritable
high similarity in VNTR patterns= closely related
creating a genetic fingerprint
DNA extracted from tissue and amplified using PCR
Restriction enzymes use to cut DNA into fragments at points near VNTR sequences
gel electrophoresis separates fragments by size and they are denatured to produce single strands
specific radioactive/ fluorescent probes bind to complementary VNTR sequences
Positions of probes are revealed→ makes pattern of DNA bands unique to individual
uses of genetic fingerprinting technology
paternity testing
identifying suspects from crime scene DNA
supporting criminal convictions with match probability calculations
identifying risk of genetic disorders and predicted severity
selecting desirable traits in selective breeding
evaluating genetic diversity by comparing variety of genetic fingerprints within a population.
limitations of genetic fingerprinting
environmental contamination can compromise results
close genetic relatives could have similar fingerprints
assumptions about variation underpin prob. calculations→ not always prove guilt or causation
polymerase chain reaction
a method for amplifying DNA fragments rapidly and efficiently
does not require living cells to rapidly replicate specific DNA fragments
also known as in vitro cloning
components required for PCR
DNA fragment→ target DNA template sequence that needs to be replicated
Primers→ short sequences of nucleotides that attach to start and end of fragment
DNA polymerase→ must be able to withstand high temps e.g. Taq polymerase
Free nucleotides
Thermocycler→ device that precisely heats and cools PCR mixture to facilitate reaction
stages of PCR
Separation (denaturation)→ 95°C:
separates hydrogen bonds between two complementary strands
Addition of primers (annealing)→ 55°C:
H bonds form between primer and starting points on each of the strands
DNA synthesis (extension)→ 72°C:
DNA polymerase adds free nucleotides to ends of primers, extending DNA to form complete copy
Advantages of PCR
rapid speed
Highly precise
Low DNA needs
No cells needed
uses of DNA sequencing
Computational biology, bioinformatics and genomics
genome analysis
genome comparison
synthetic biology
bioinformatics
Involves developing software, computing tools and mathematical models to collect, store and analyse biological datasets e.g. nucleotide sequences of genes and genomes
computational biology
uses bioinformatics and biological data to model systems and processes
genomics
studying genomes of organisms
genome analysis
can be used to study human health and disease
can identify patterns in DNA and disease risks
Pathogen genomes can be sequenced:
identifying sources and transmission roots of diseases
detecting antibiotic resistant strains
developing new treatments and vaccines
monitoring disease outbreaks
comparing genomes
indicates common ancestry using similarities in DNA barcoding:
Advantages of DNA barcoding:
fast and affordable sequencing
classification of species
estimating evolutionary divergence times
genomics and proteomics
genomics→ the study of genomes using DNA sequencing
Proteomics→ examines complete set of proteins produced by genome, including structure and function
number of proteins can exceed number of genes (e.g. mRNA splicing, post-translational modifications)→ makes relationship between genotype and phenotype complex
synthetic biology
design and construction of new biological parts, pathways and organisms
can be used for:
synthesising functional genes to replace faulty ones as treatments for genetic disorders
utilising microorganisms and biological systems to produce drugs in efficient manner
constructing artificial genomes
what is genetic engineering
the deliberate manipulation of genetic material to modify an organism’s characteristics
often involves gene transfer
what is recombinant DNA
DNA that is altered to contain nucleotides from 2 different organisms
allows DNA fragments to be transferred between organims
organisms that receive transferred DNA fragments are called genetically modified/ transgenic organisms
stages in gene transfer
desired gene is identified and isolated
multiple copies of the gene are made using PCR
gene inserted into vector
vector delivers gene into cells in a different organism
cells with new gene identified e.g. by using marker
cells with new gene are cloned
methods of producing DNA fragments
making complementary DNA (cDNA) using reverse transcriptase and mRNA
Cleaving DNA from a donor organism with restriction enzymes
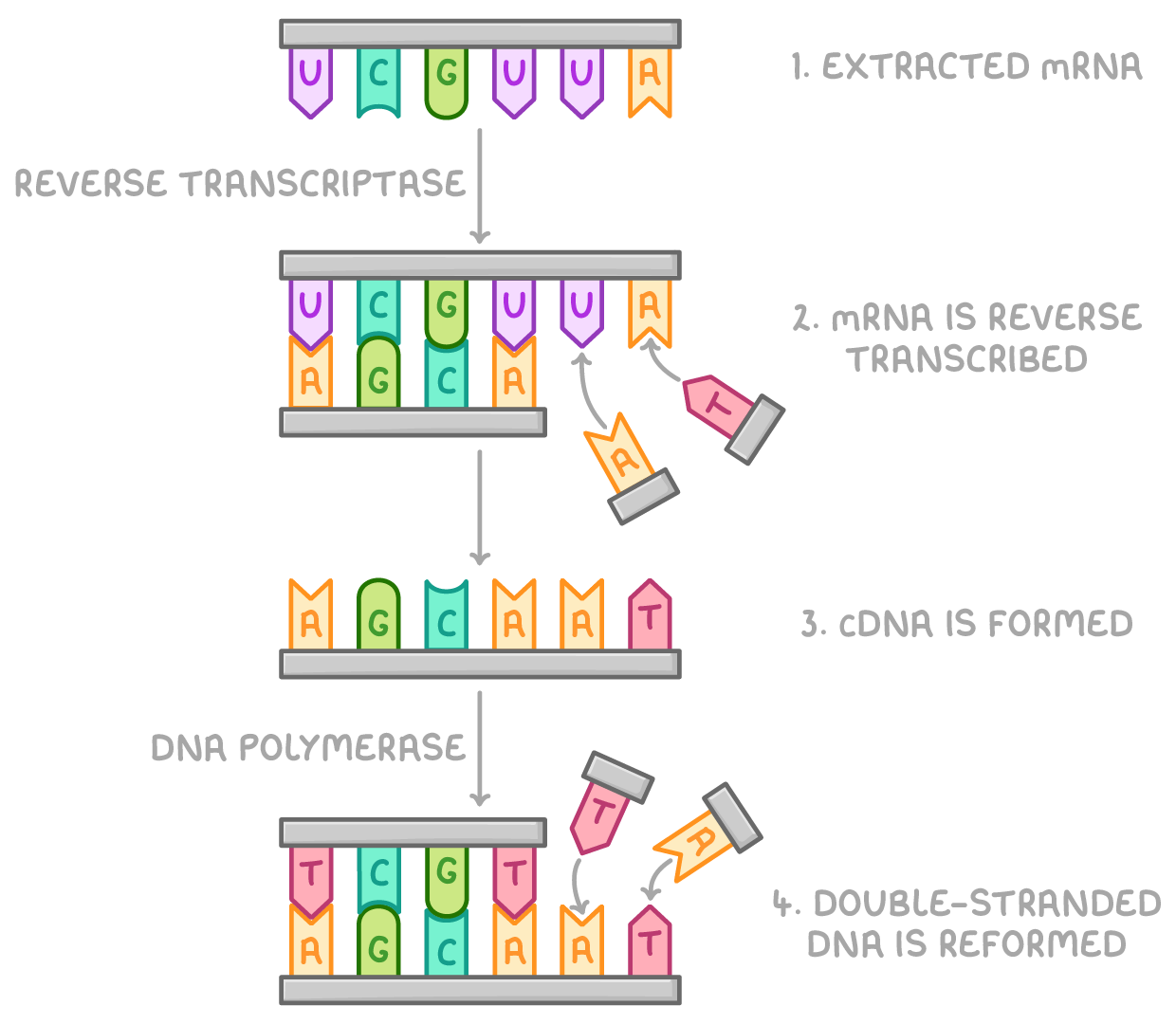
how does reverse transcriptase produce DNA fragments
mRNA is extracted from cells
mRNA is reverse transcribed using reverse transcriptase enzyme and DNA nucleotides
makes a cDNA strand identical to original DNA strand and cDNA is isolated from mRNA strand
cDNA, free nucleotides and DNA polymerase can form other strand of DNA, reforming desired gene
using restriction enzymes to cut DNA
restriction endonuclease used
can recognise and cut DNA at specific palindromic nucleotide sequences to isolate gene fragments
process of using restriction enzymes
DNA incubated with chosen restriction enzyme
restriction enzyme identify palindromic sequences in DNA double helix and cut double stranded DNA if their recognition sequence is present- allow enzyme to separate fragment from rest of the strand.
Enzymes cut target gene fragment via hydrolysis
different restriction enzymes cut at different sequences based on their active site shape
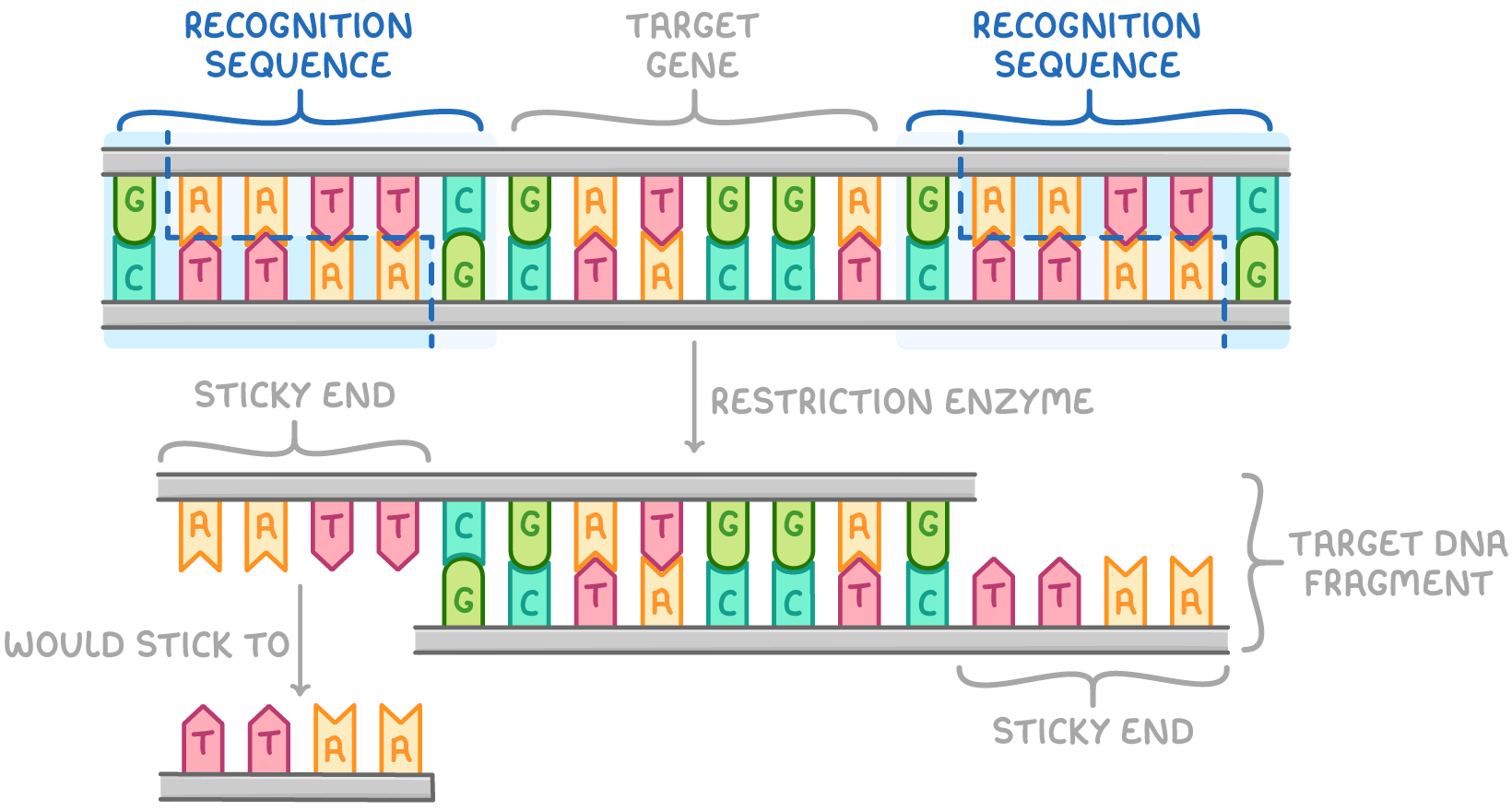
sticky ends
short overhanging sequences of unpaired bases that can bind to other DNA fragments when they are inserted into vectors
often found DNA cut by restriction enzymes
steps in forming recombinant DNA
inserting DNA fragments into vectors
transferring recombinant DNA into host cells
identifying transformed host cells
in vivo cloning/ in vivo DNA amplification
process of producing large quantities of a target DNA fragment in living cells
inserting DNA fragments into vectors
vector cut open at specific site using restriction enzyme- creates sticky ends
same restriction enzyme used to cut target DNA fragment, creating complementary sticky ends
DNA ligase forms phosphodiester bonds between sugar and phosphate groups on two strands of DNA- joins sticky ends of vector and DNA together
newly formed combined DNA molecule= recombinant DNA
transferring recombinant DNA into host cells
can be done using:
plasmid vectors
bacteriophage vectors
plasmid vectors
small, circular DNA molecules typically found in bacteria
host cells treated to enhance uptake of plasmids that have recombinant DNA
e.g. applying Ca2+ and temp. shifts= bacterial membranes more permeable to plasmids
electroporation uses electric current to make bacterial membranes more porous- helps plasmids enter bacterial cells more easily
bacteriophage vectors
viruses that infect bacteria
inject their DNA into host bacterial cells during infection
phage DNA, now carrying recombinant DNA, inserts into hosts DNA
identifying transformed host cells
not all host cells may uptake DNA- important to identify which cells have been transformed successfully
marker genes indicate which host cells took up recombinant DNA
Inserted into vectors alongside target genes
transformed cells cultivated on selective agar plates
only transformed cells display characteristics encoded by marker genes
transformed cells can be cultured to mass produce target DNA fragment through cellular replication
types of marker genes
for a specific trait e.g. antibiotic resistance, ensures only transformed cells form colonies
visible under UV light like green fluorescent protein
inserting marker gene within green fluorescent protein gene inhibits fluorescence if it is successfully incorporated
marker gene coding for an enzyme that alters colour of a specific substrate
genetically modified crops
can make them resistant to herbicides or insects
e.g. soy beans have been modified to include gene that produces protein that is toxic to many insect pests but is harmless to humans
advantages of GM crops (5)
less labour than traditional herbicides and pesticides
cheaper than traditional herbicides and pesticides
herbicide resistant GM crops allow use of herbicides to kill weeds without harming the crop, increasing yield
insect resistant GM crops less likely to be killed by pests
insect resistant Gm crops reduce need for pesticides- better for environment
disadvantages of GM crops (5)
encourage monocultures= less biodiversity
herbicide resistant GM plants may crossbreed with wild plants- makes herbicide resistant weeds
loss of traditional crop varieties reduces genetic diversity- makes crops vulnerable to disease/ climate changes
non-target insects could be harmed by toxin coded for by insect resistant GM crops
insect pests may evolve resistance to toxins
advantages of GM animals
improves quality e.g. enhancing disease resistance
improves quantity e.g. enable year-round reproduction
improves productivity e.g. faster growth
pharming
production of pharmaceuticals and human medicines by inserting human genes into other animals
allows for pharmaceutical proteins to be extracted from GM animals’ milk or blood at high yields
advantages of pharming
enables production of rare treatments
makes drugs more accessible
disadvantages of pharming
animal welfare concerns
can lead to animals being viewed solely as commodities
creating GM microorganisms
can be used in development of new treatments for diseases e.g. poliovirus can be engineered to target cancer
advantages of GM pathogens
offers potential treatments for diseases previously deemed incurable
can produce essential medicines e.g. insulin
useful in creating vaccines
facilitates creation of DNA libraries for research
disadvantages of GM pathogens
carries risk of accidental infections and disease outbreaks
danger that engineered pathogens could revert to original harmful form
could be misused in biological warfare
ethical concerns with GMOs
legal patenting of engineering engineered organisms raises questions about global access
especially affects smallholder farmers in developing countries who may be unable to afford patented seeds due high costs and legal barriers
how does gene therapy treat genetic disorders
identify abnormal gene responsible for disorder
engineer normal, functional version of this gene by removing it from health cells or synthesising it in lab
deliver normal allele to nuclei of target cells using vector
ensure gene is successfully integrated into cells’ DNA and expressed correctly
main approaches to using gene therapy
counteracting recessive disorders:
add functional dominant alleles
silences non-functional recessive alleles
silencing faulty dominant alleles
insert DNA sequences that inactivate harmful dominant alleles
prevents dominant allele functioning properly and causing harm
somatic gene therapy
replaces mutant alleles with healthy alleles in affected somatic (body) cells to treat diseases
alters somatic cells e.g. lungs in cystic fibrosis
impacts specific tissues and organs only
genetic modifications not inherited by offspring
germline gene therapy
involves inserting healthy allele into germ cells or embryos to prevent genetic diseases from birth
alters egg and sperm cells or embryos
influences all cells within body when inherited
modifications be passed down to future generations
ethical benefits of gene therapy
extends lives by treating diseases
enhances quality of life
germline GT= carrier parent can have children free from genetic disorders
reduces overall disease burden in population
ethical issues of gene therapy
potential misuse for enhancing aesthetic attributes rather than medical need
risk of causing unintended harm
diverts scarce healthcare resources
high cost- restricted access?
issues with somatic gene therapy
delivering health alleles to cells is challenging
getting healthy alleles into nucleus is challenging
maintaining the expression of healthy alleles is challenging
effects are short term as somatic cells have a limited lifespan and are replaced by cells with faulty allele
issues germline gene therapy
the rights of unborn child are violated as they cannot provide consent
it causes irreversible changes, long term outcome of which aren’t fully understood
it could used for non- therapeutic enhancements such as selecting desirable traits