Electronic Structure and Periodic Properties of Elements
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What speed does all electromagnetic radiation travel at?
The speed of light (c= 3x10^8m/s)
What are the three characteristics of waves?
1. Wavelength (λ)
2. Amplitude
3. Frequency (v - nu not a v)
What is the relation between frequency and wavelength?
c = λv
the higher the frequency, the smaller the wavelength as the speed of light is a constant.
What is the difference between visible light and electromagnetic radiation?
Visible light is a part of the spectrum of electromagnetic radiation, but not all electromagnetic radiation is visible light
What are the two kinds of interference that act on waves?
Constructive and Destructive interference
What feature do standing waves have?
They are constrined in some region of space, so they have nodes (fixed points with no amplitude)
What can wave theory not explain? what does explain this phenomenon?
Blackbody radiation, where hot objects emit light depending on their temperature. Planck proposed that energy can only be absorbed or released from atoms in certain amounts called "quanta"
Define a quantum
the smallest amount of energy that can be emitted or absorbed as electromagnetic radiation
What is the relationship between energy and frequency?
E = hv, h being the planck constant
Why don't the wave-like properties of matter seem noticeable in our daily lives?
Due to our relative size to the quantum world, the effects are too minute for us to notice on the large scale.
What would you expect to see when you shine a certain speed of light onto a metal surface?
If the frequency of the light is high enough, then you would observe the photoelectric effect. This is when the metal surface absorbs light and then emits electrons
What is the energy of one photon? Who proposed the idea that particles could have wave-like properties?
E = hv = hc/λ, Einstein
What is a continuous spectrum?
It is the spectrum produced when the radiation from a light source is separated into its different wavelength components
What is the difference between a continuous spectrum and the spectrum you would see from the light of an excited gaseous element?
A continuous spectrum has a rainbow of continuous colours, while the spectrum of an excited element will be a line spectrum. There will be discontinuous lines of different colours.
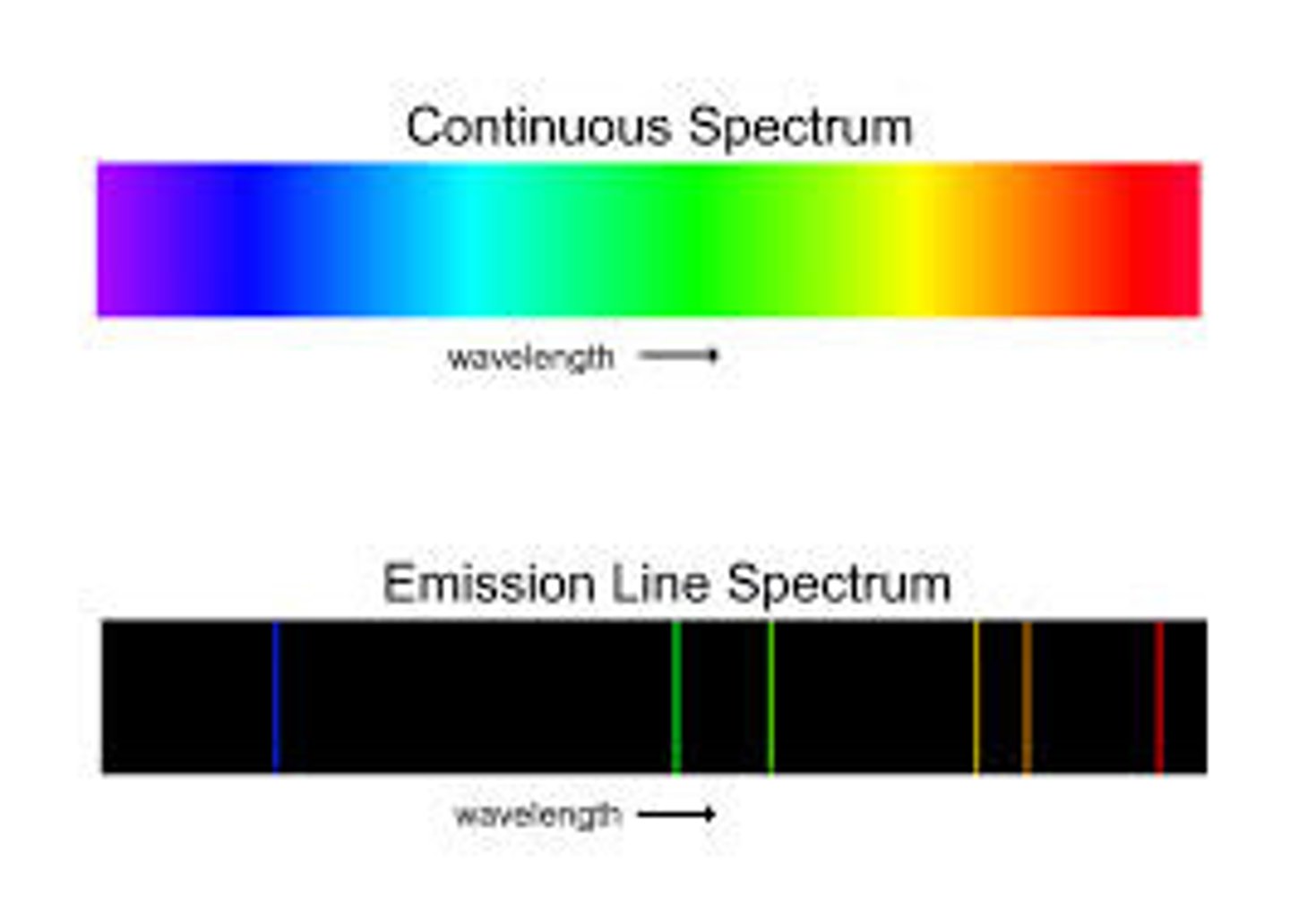
Who assumed that electrons in an element were confined to certain energy states? What were his three postulates?
Niels Bohr
1. Only orbits of specific radii are permitted for electrons in an atom, they correspond to certain definite energies
2. An electron in a permitted orbit has a specific energy, called an "allowed" energy state
3. Energy is only emitted or absorbed by an electron jumping between one allowed energy state to another, and this energy is lost or gained as a photon
What is photoemission?
the giving off of a photon
Why is the light given off from excited atoms quantized?
As the energy states of the electrons are quantized, so is the light emitted from the energy states
What is the formula for quantized electron energy?
ΔE = -2.18x10^-18 [(1/hf^2)-(1/hi^2)]