OCHEM: Synthesis of 4-Methylcyclohexene from 4-methylcyclohexanol
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Whats the purpose of the lab
synthesize 4-methylcyclohexene from 4-methylcyclohexanol (which is a secondary alcohol)
What type of reaction is observed
an acid dehydration of a secondary alcohol
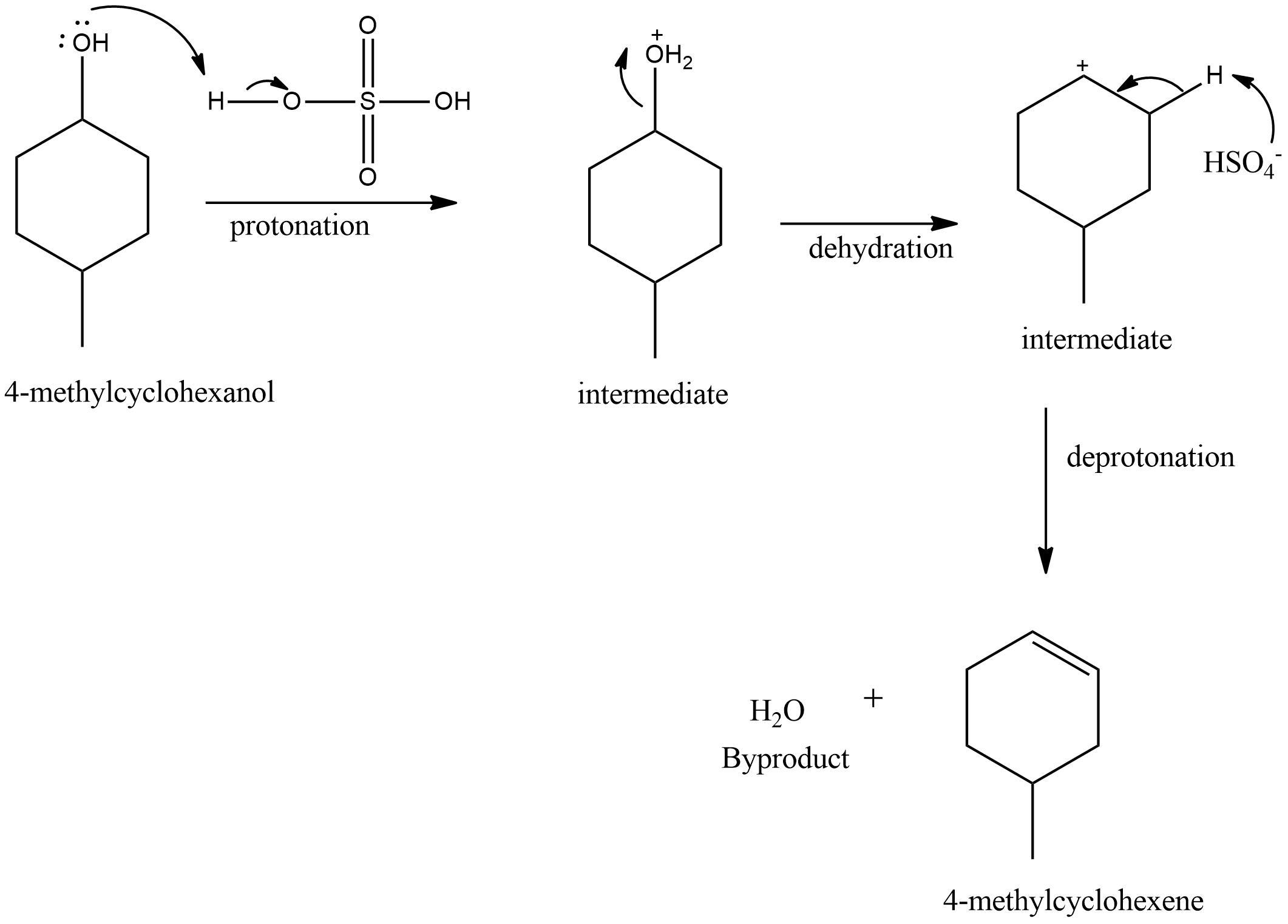
why do we add phosphoric acid to sulfuric acid
phosphoric acid reduces the extensive charring from just using sulfuric acid
a bit of sulfuric acid is used to make reaction proceed faster
What does the separatory funnel have
the top layer is the organic layerand the bottom layer is the aqueous layer.
what is the purpose of the saturated NaCL in the separatory funnel?
to remove large amounts of dissolved water and to minimize the amount of the organic product that dissolves in the aqueous layer
What layer has been extracted after using separatory funnel technique
organic top layer was extracted
whats the purpose of anhydrous sodium sulfate
a drying agent that removes minute particles of water from organic solvent
why do we perform two distillation processes
to get the product from the reaction mixture & work with Le Chatliers’s principle
to purify product
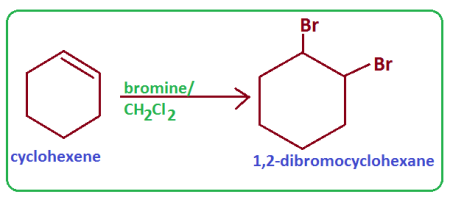
why do we test the presence of cyclohexene with bromine
its a saturation test to test if the product produced contained a double bond
if it goes from red to colorless, then theres the presence of a double bond
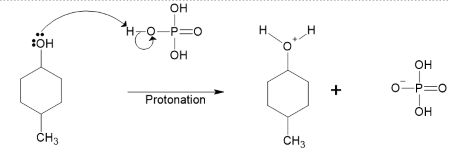
mechanism for dehydration of 4-methyl cyclohexanol catalyzed by phosphoric acid
cyclohexanol dehydration
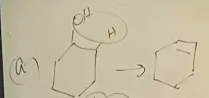
1-methylcylohexanol dehydration
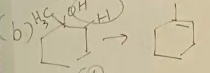
2-methylcyclohexanol
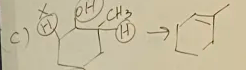
2,2-dimethylcyclohexanol
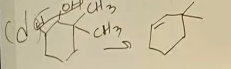
1,2-cyclohexanediol dehydration