Carbon Chains
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
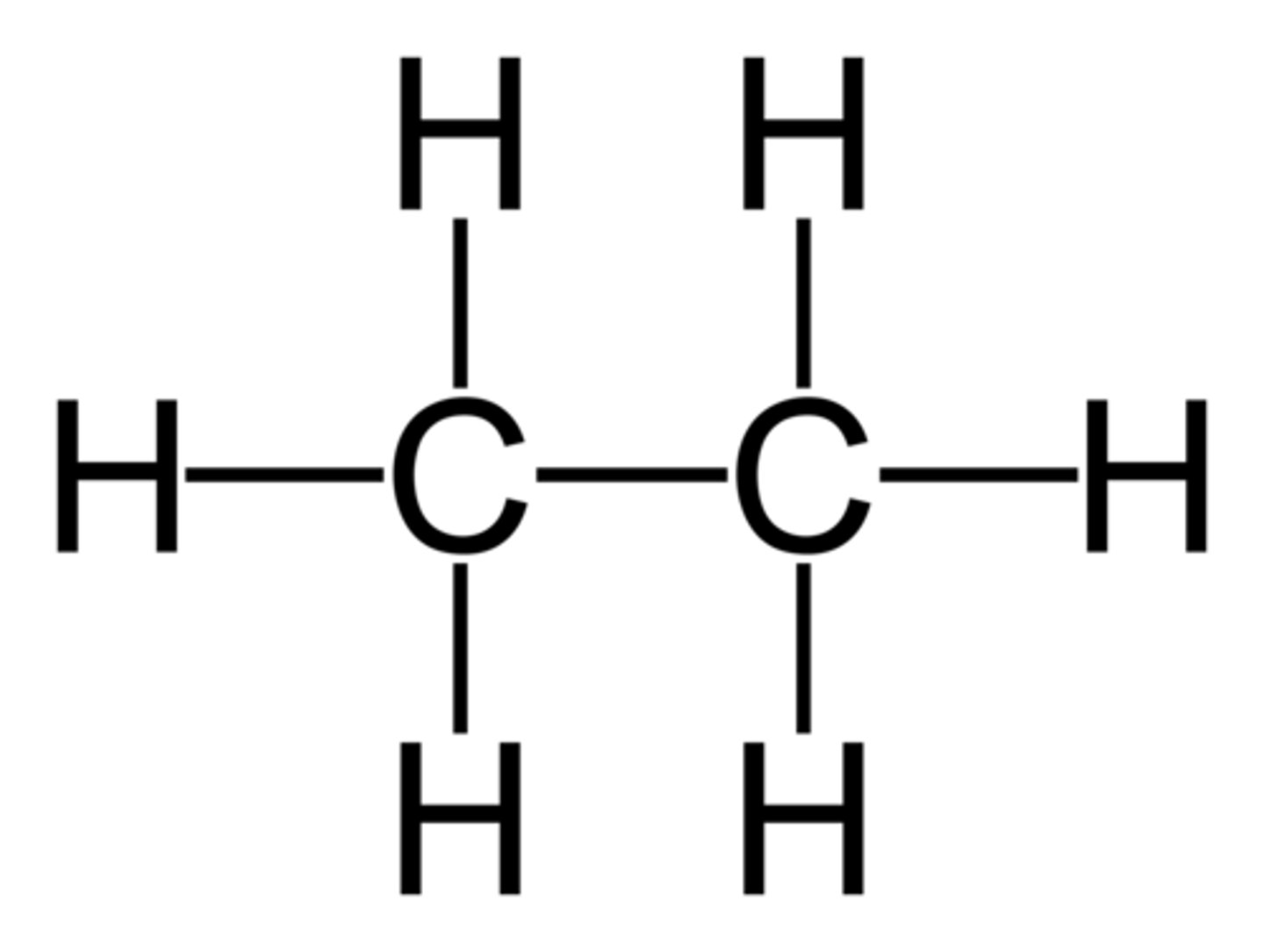
alkane
- nonpolar (C-C, C-H)
- does not hold H-bond
C - C (single bonded)
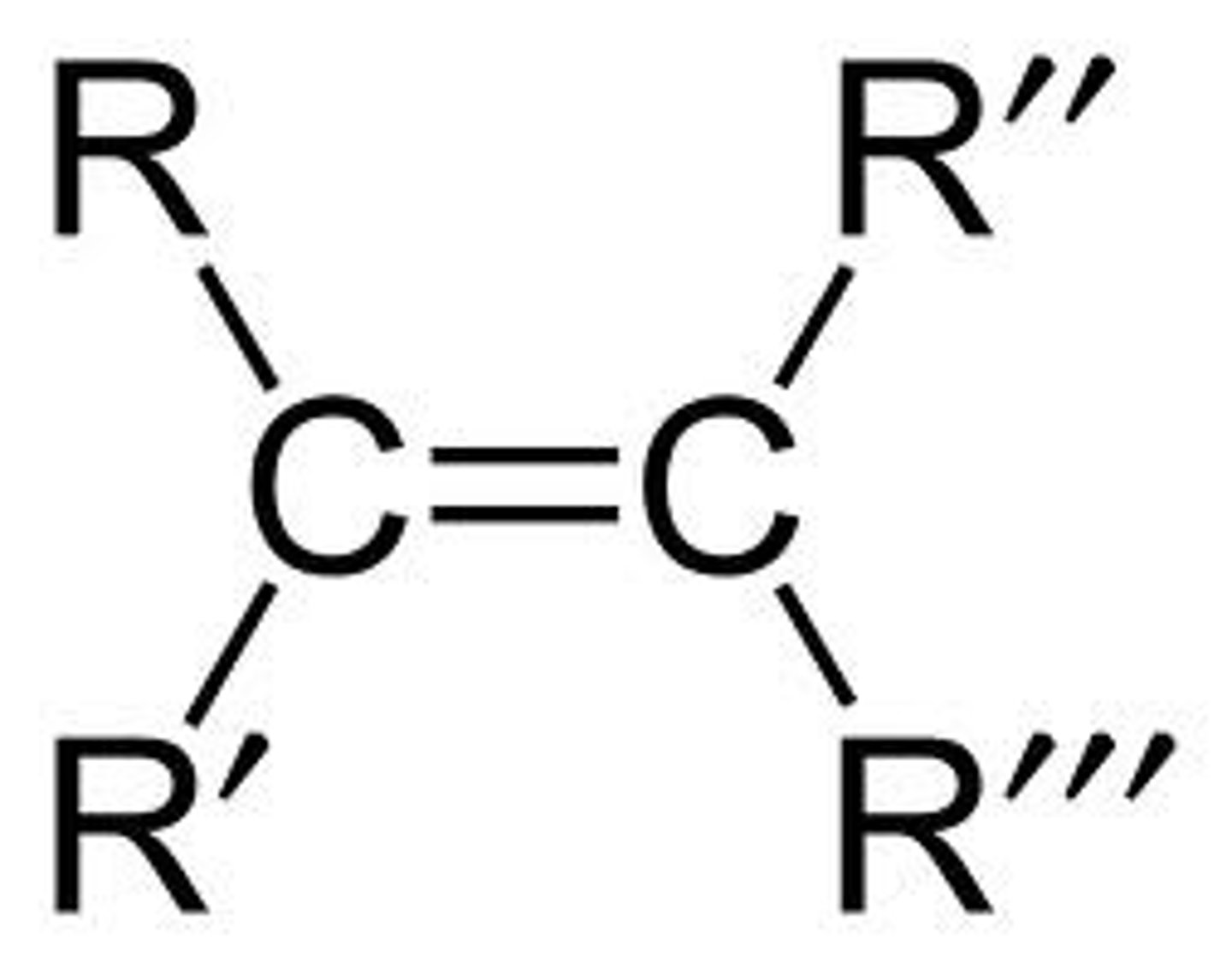
alkene
- nonpolar (C-C, C-H)
- does not hold H-bond
C = C (double bonded)
alkyne
- nonpolar (C-C, C-H)
- does not hold H-bond
C (triple bonded) C
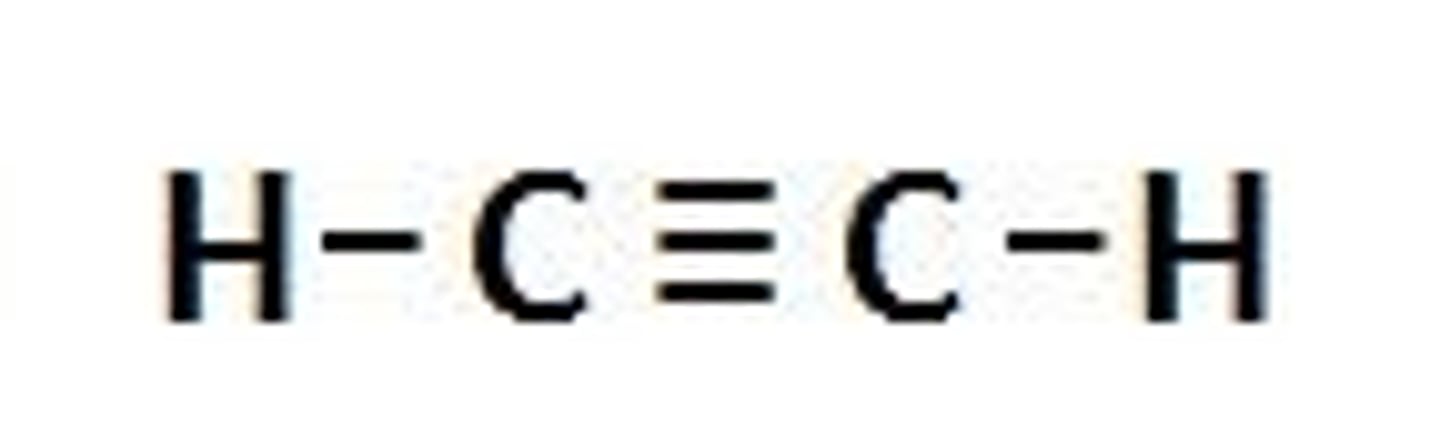
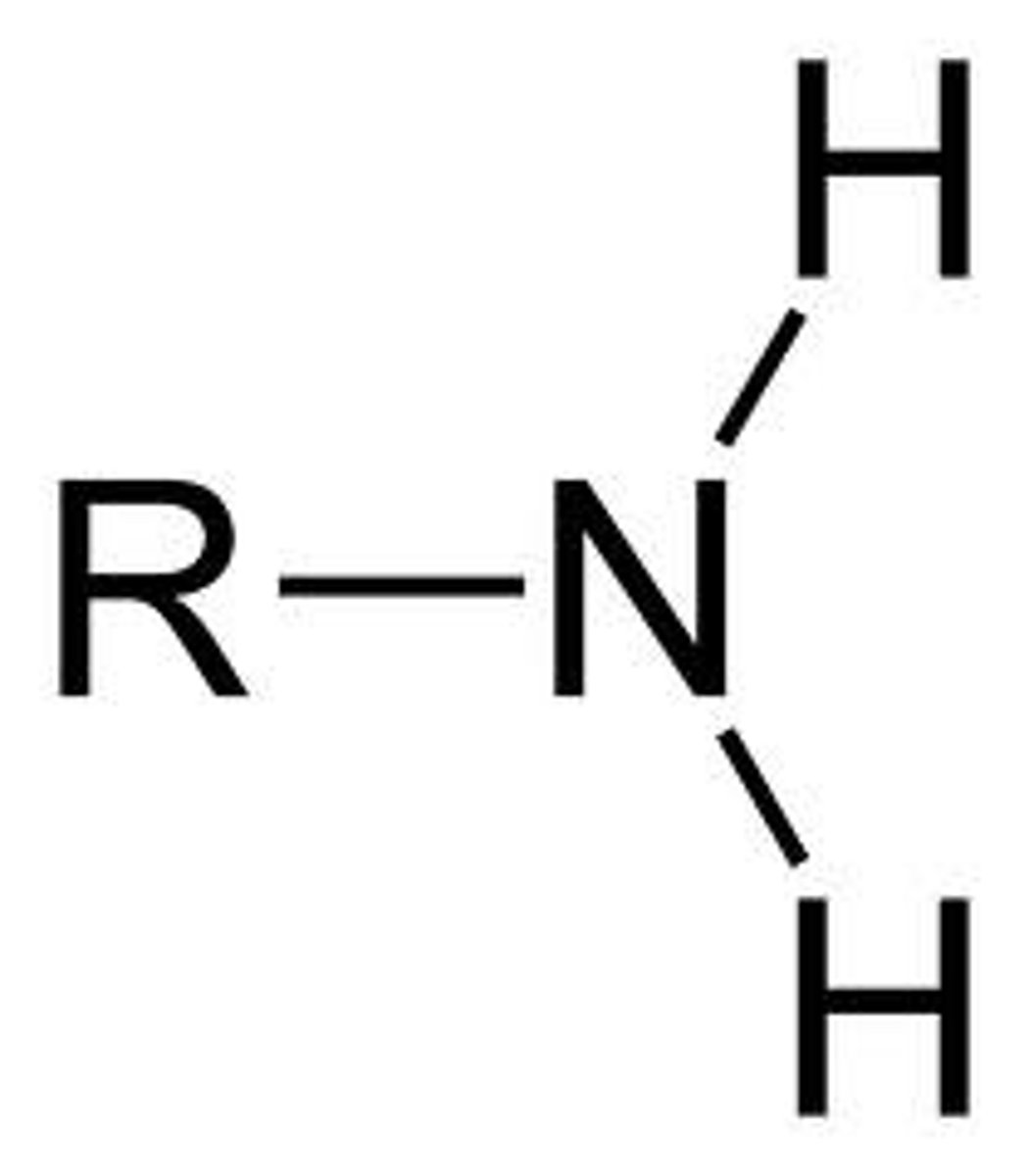
amine
- introduces polar bonds (C-X, C-N, C-O)
- dipole-dipole or H-bonding interactions
C - NH_2
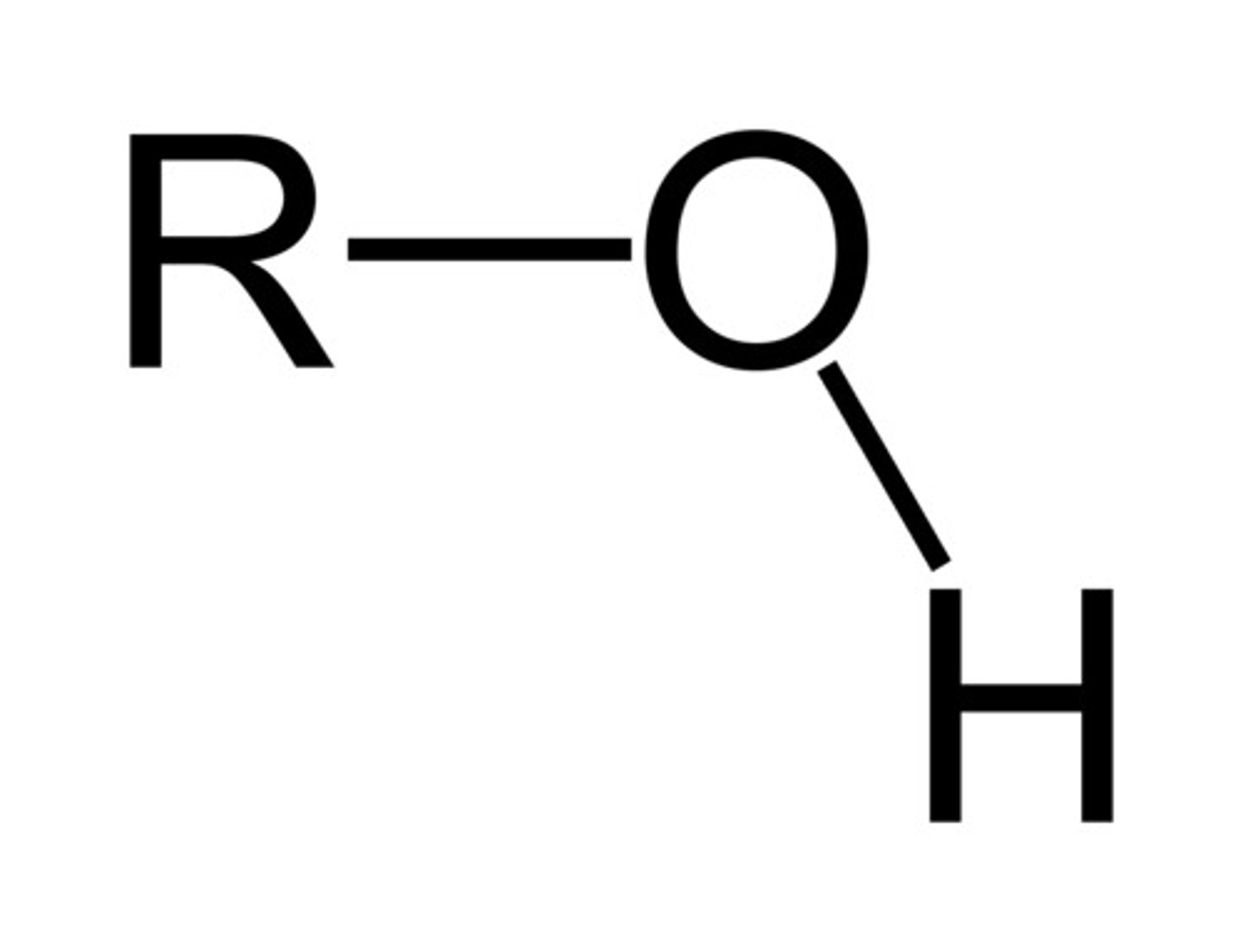
alcohol
- introduces polar bonds (C-X, C-N, C-O)
- dipole-dipole or H-bonding interactions
C - OH ; applies to those ending in "ol"
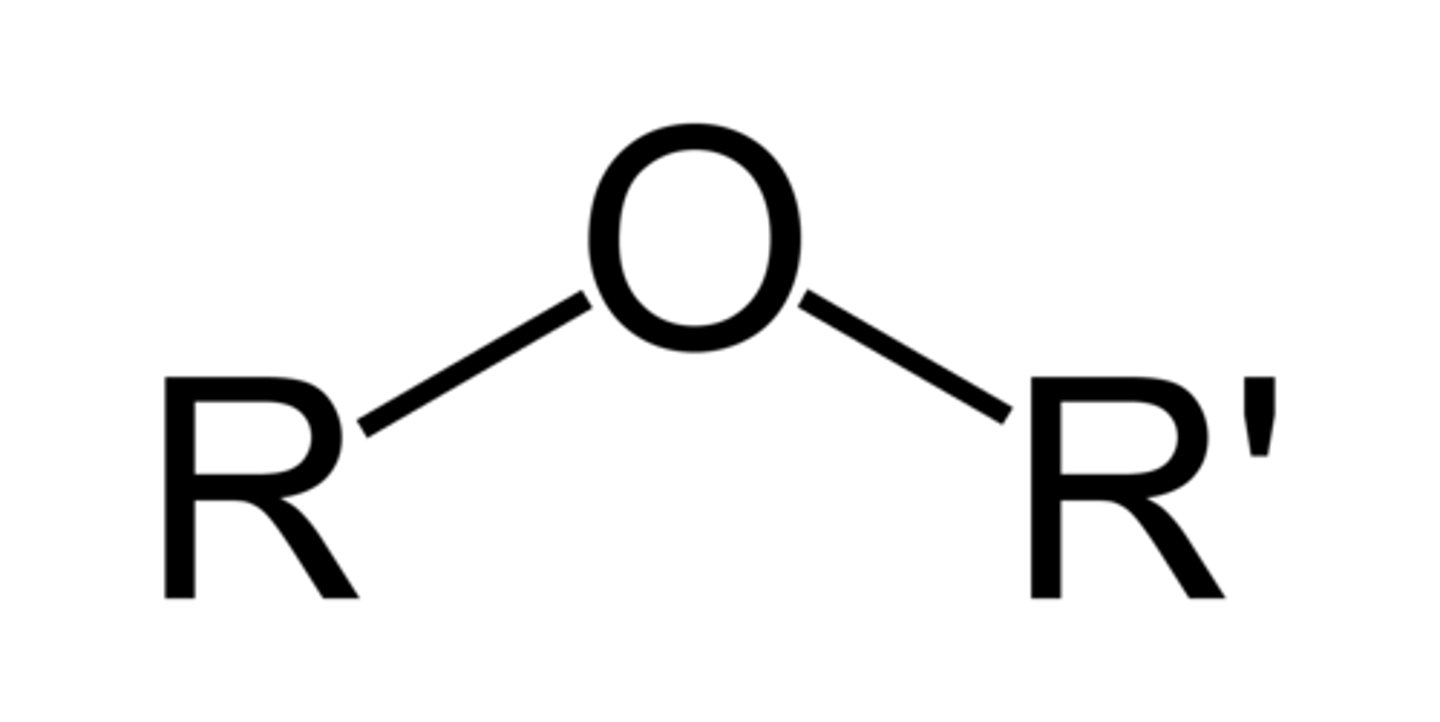
ether
- introduces polar bonds (C-X, C-N, C-O)
- dipole-dipole or H-bonding interactions
C - O - C
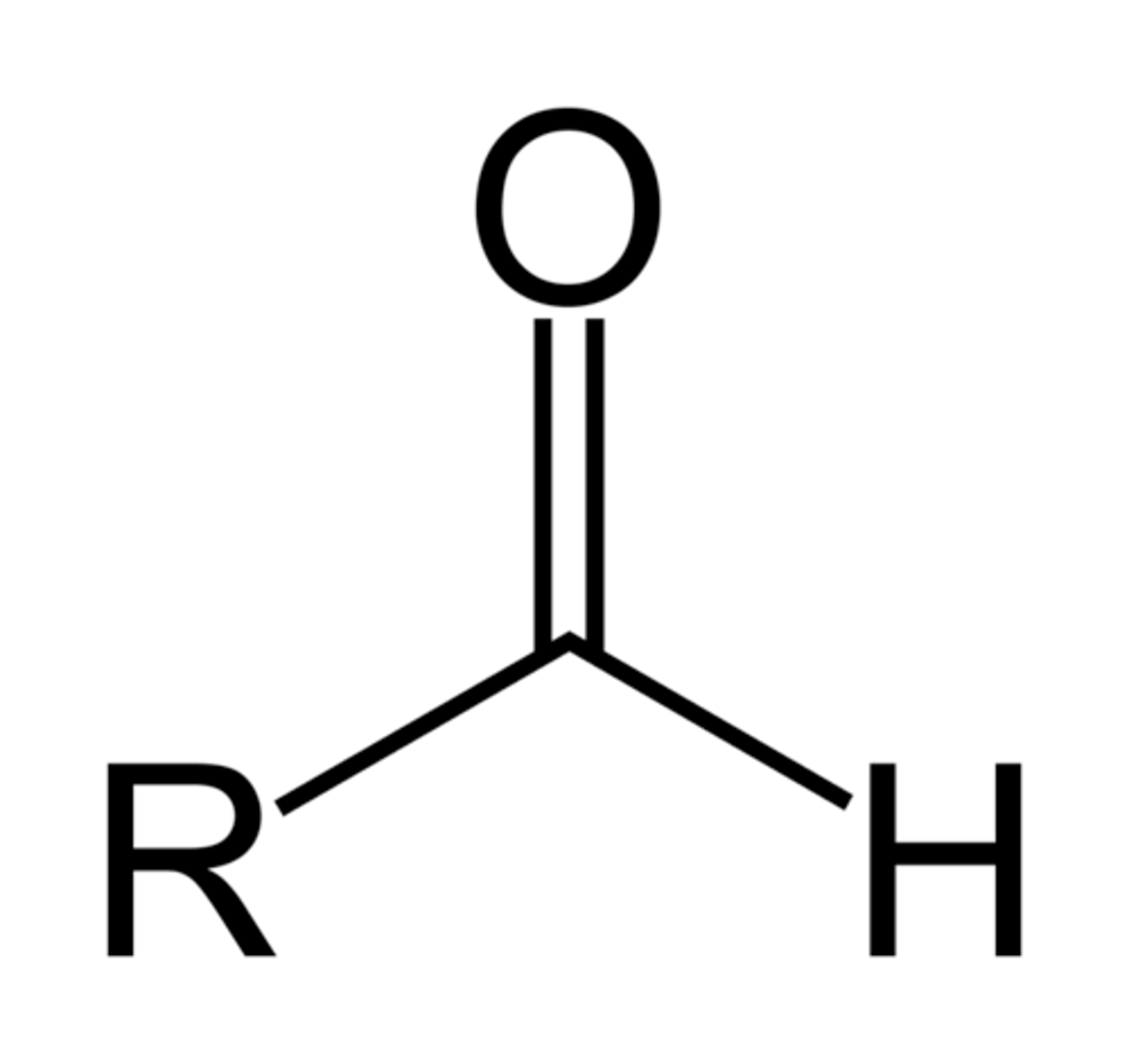
aldehyde
- polar bonds (C-O, C=O, O-H, C-N, N-H)
- can participate in H-bonds with water or alcohols
H - C = O (terminal group)
ketone
- polar bonds (C-O, C=O, O-H, C-N, N-H)
- can participate in H-bonds with water or alcohols
C = O (non-hydrogen)
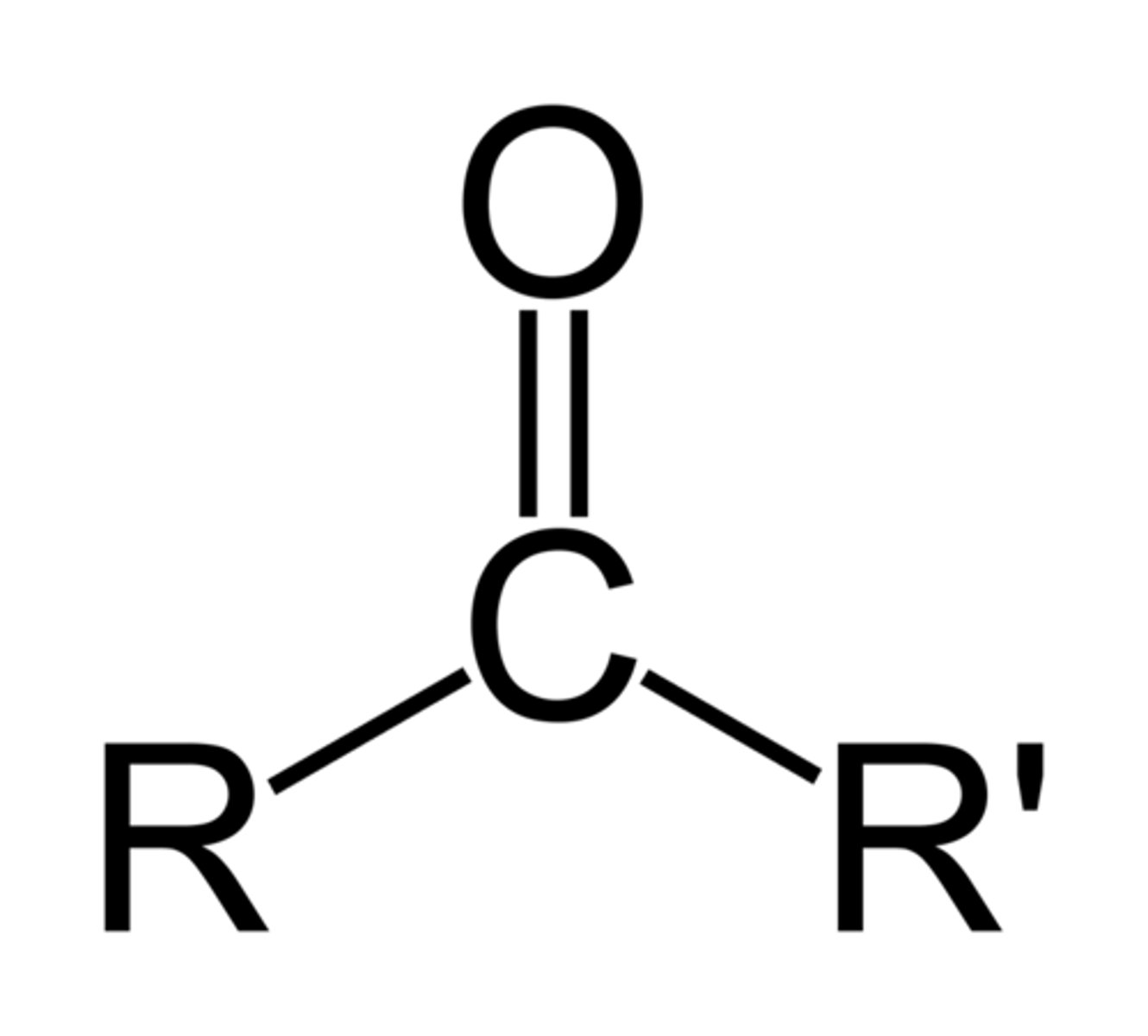
carboxylic acid
- polar bonds (C-O, C=O, O-H, C-N, N-H)
- can participate in H-bonds with water or alcohols
OH - C = O (NOT alcohol; stronger H_2 bond)
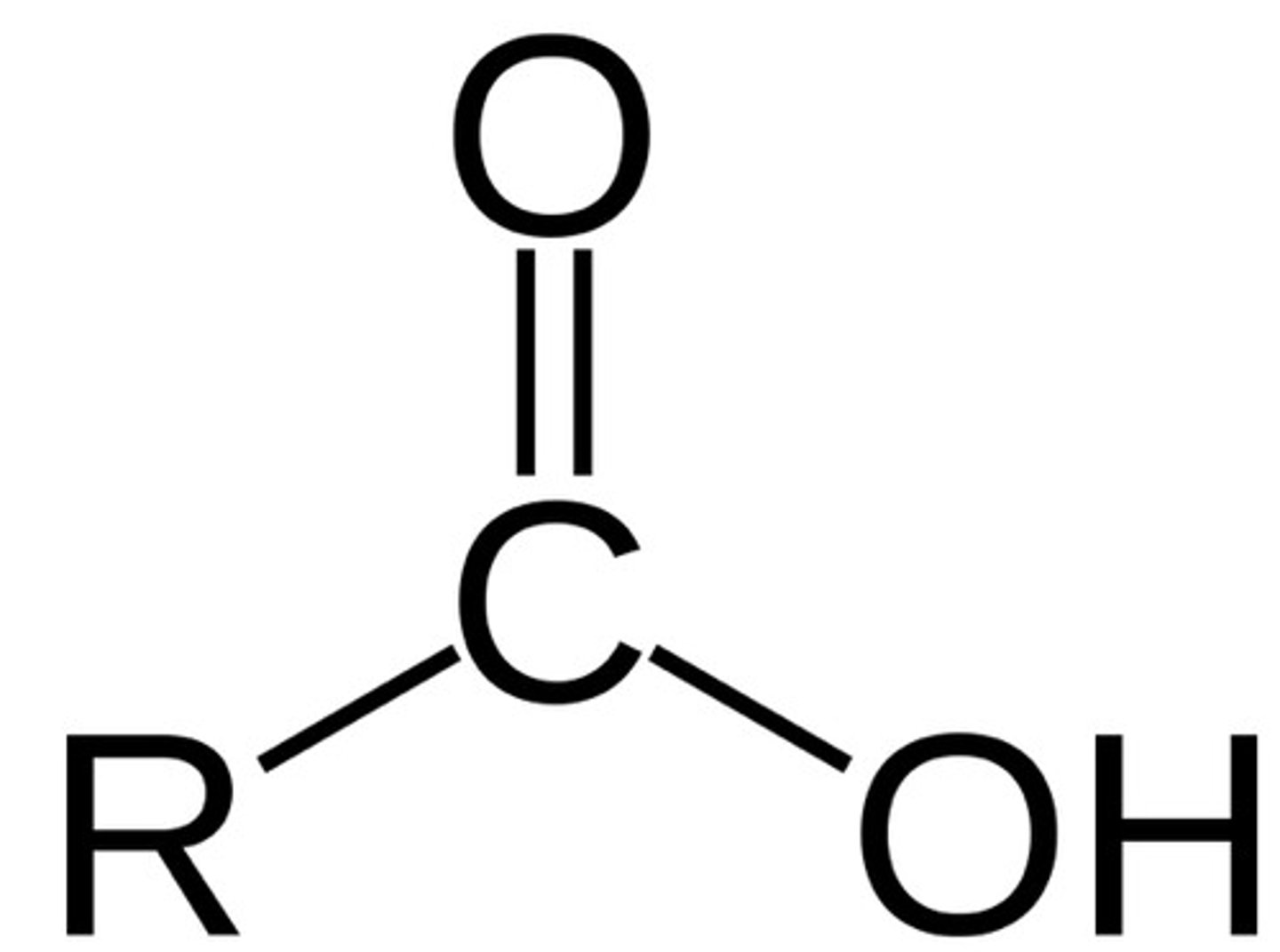
ester
- polar bonds (C-O, C=O, O-H, C-N, N-H)
- can participate in H-bonds with water or alcohols
O - C = O (multiple O)
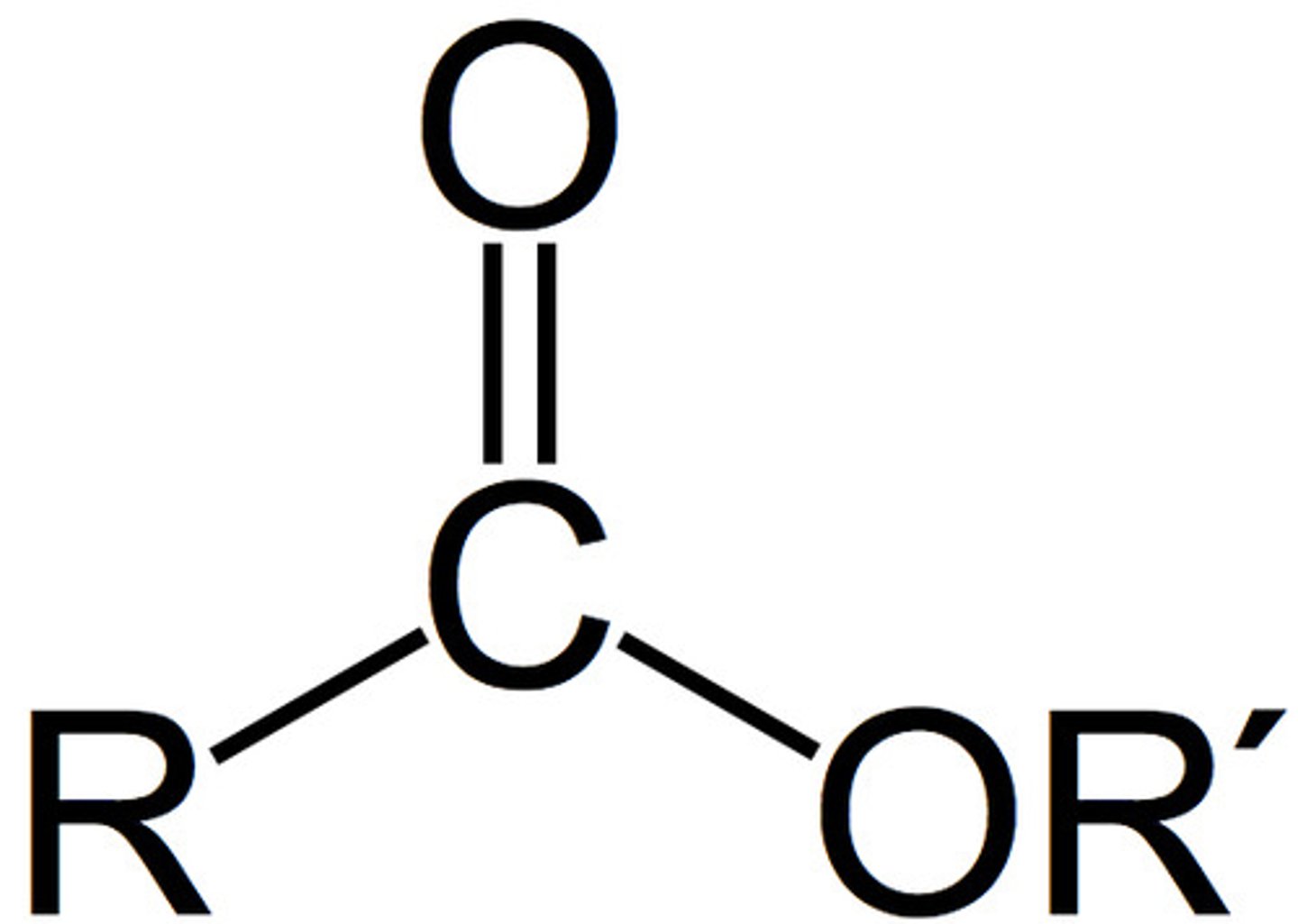
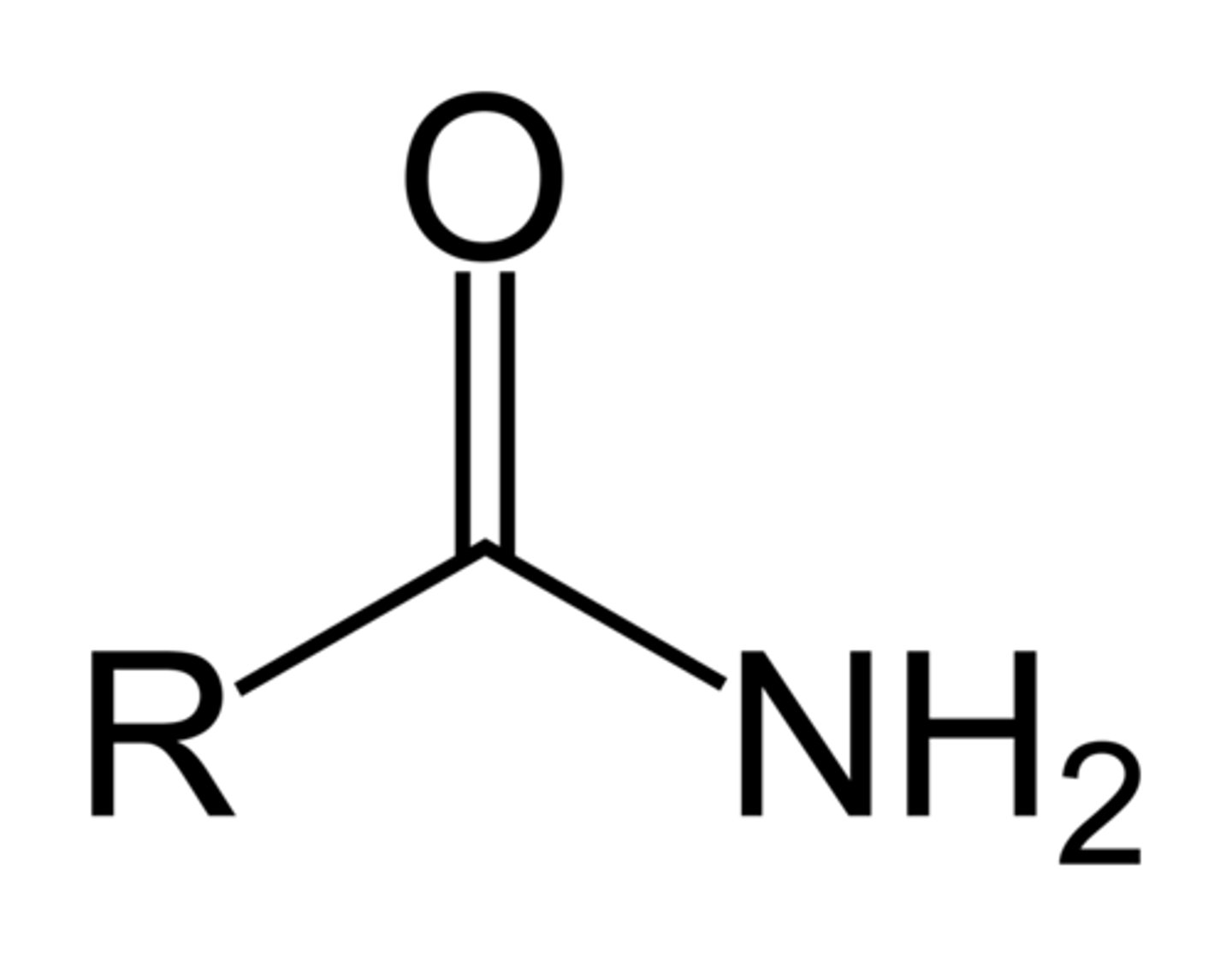
amide
- polar bonds (C-O, C=O, O-H, C-N, N-H)
- can participate in H-bonds with water or alcohols
NH_2 - C = O (w/ double to O)