Microbiology Lecture Exam 3 (Fixed)
1/166
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
167 Terms
What Is a Virus?
• Virus: genetic element that can multiply only in a living (host) cell
– Not living, not found on tree of life
– Obligate intracellular parasite: Needs host cell for energy, metabolic intermediates, protein synthesis
• Replication/reproduction occurs only upon infection (entry into host cell).
Viral Shapes
•helical - as in tobacco mosaic virus
•Spherical viruses – (HIV, influenza, coronavirus)
•Filamentous viruses – (Marburg, Ebola)
•Many viruses incorporate other structures such as heads, tails, and spikes (bacteriophages).
Viral Components
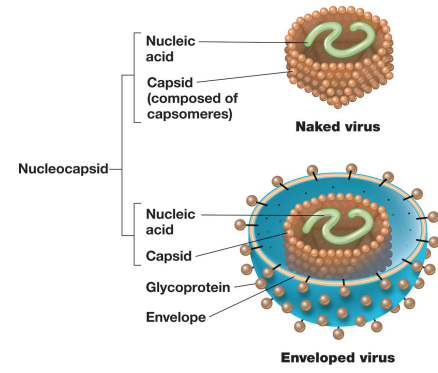
– Capsid: the protein shell that surrounds the genome of a virus
– Naked viruses (e.g., most bacterial and plant viruses) have no other layers. (^no enevelope)
– Enveloped viruses (e.g., many animal viruses) have an outer layer consisting of a phospholipid bilayer (from host cell membrane) and viral proteins.
▪ Nucleocapsid: nucleic acid + protein in enveloped viruses (all viruses)
– Virion surface proteins important for host cell attachment and may include enzymes involved in infection/replication
^1st step in infection
Fomites
inanimate object that transmits diseases
The protein coat is the?
•The protein coat is the capsid
•Capsid is made of capsomeres
•Some capsids are surrounded by envelopes. (Includes components from host cell.)
Complete virus particle is called a
•Complete virus particle is called a Virion
The ________ is the viral genome and the capsid?
The NUCLEOCAPSID is the viral genome and the capsid
Naked viruses tend to be _____ resistant physically than enveloped viruses.
•Naked viruses tend to be more resistant physically than enveloped viruses.
•Enveloped viruses can trick the immune system.
Enveloped vs Naked Structure
The host range refers to?
•The HOST-RANGE refers to the spectrum of hosts that a particular virus is capable of infecting
-What host can it affect?
Viral Specificity refers to?
•VIRAL SPECIFICITY refers to the specific cell types within an organism that a virus can infect
-What tissue can it affect?
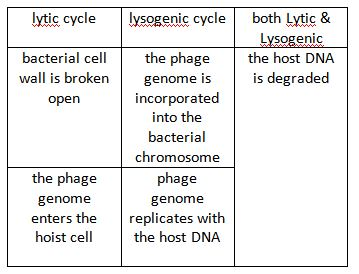
virulent (lytic) infection
– virulent (lytic) infection: replicates and destroys host
▪ Host cell metabolism redirected to support multiplication and virion assembly

lysogenic infection:
– lysogenic infection: host cell genetically altered because viral genome becomes part of host genome
cytopathic effect
Cytopathic effect (CPE) refers to the visible changes in the morphology and function of cells caused by viral infection
Kinds of Virus Symmetry?
▪ helical symmetry: rod-shaped (e.g., tobacco mosaic virus or TMV) – length determined by length of nucleic acid
– width determined by size and packaging of capsomeres
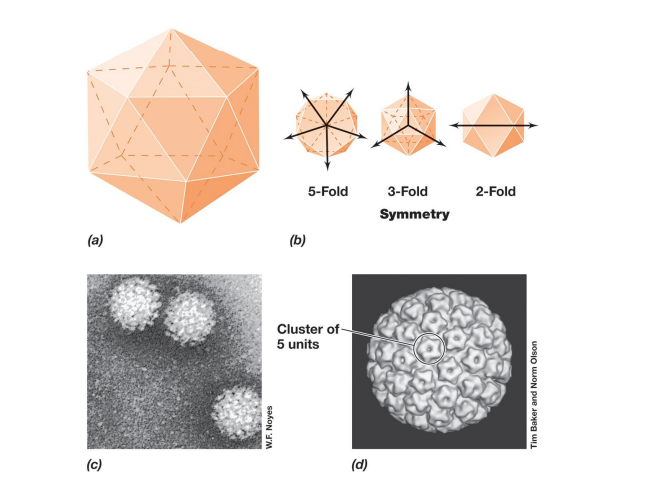
▪ icosahedral symmetry: spherical (e.g., human papillomavirus;
– 20 triangular faces, 12 vertices; 5, 3, or 2 identical segments
– most efficient arrangement of subunits in a closed shell
– requires fewest capsomeres
– Some structures highly
▪ Most complex are head-plus-tail bacteriophages
Viral Diversity
– either DNA or RNA genomes
– single-stranded or double-stranded
Viruses can be classified on the basis of?
– Viruses can be classified on the basis of the hosts they infect
Are all viruses negative?
– Not all virus have negative effects; sometimes beneficial
▪ e.g., Arabidopsis infected with plum pox virus increases drought tolerance
▪ e.g., insect densovirus infection of rosy apple aphid results in decreased size and offspring, but wings form
▪ e.g., hepatitis G coinfection of HIV patients decreases HIV replication and infectivity
Most viruses are smaller than?
Most viruses are smaller than prokaryotic cells; ranging from 0.02 to 0.3 μm.
Capsomere
Capsomere: individual protein molecules arranged in a precise and highly repetitive pattern around the nucleic acid making up the capsid
Enveloped Viruses
– have lipoprotein membrane surrounding nucleocapsid
– relatively few enveloped plant or bacterial viruses because of cell walls surrounding cell membrane
– Entire virion enters animal cell during infection.
Enzymes Inside Virions
– lysozyme
▪ makes hole in cell wall to allow nucleic acid entry
▪ also lyses bacterial cell to release new virions
– neuraminidases (influenza)
▪ destroy glycoproteins and glycolipids
▪ allows liberation of viruses from cell
nucleic acid polymerases:
▪ Reverse transcriptase: RNA-dependent DNA polymerase in retroviruses
Bacterial viruses are ____ to grow?
• Bacterial viruses are easiest to grow (hosts in liquid medium or spread as “lawns” on agar and inoculated with virus).
• Animal and plant viruses cultivated in tissue cultures
(from animal organ in culture medium or hairy rootbased system in liquid medium).
Titer
number of infectious virions per volume of fluid
Plaque assay:
▪ Plaques are clear zones of cell lysis that develop on lawns of host cells where successful viral infection occurs
Plating efficiency
– Plating efficiency important in quantitative virology.
– The number of plaque-forming units is always lower than direct counts by electron microscopy. (b/c can’t differentiate between active and non active)
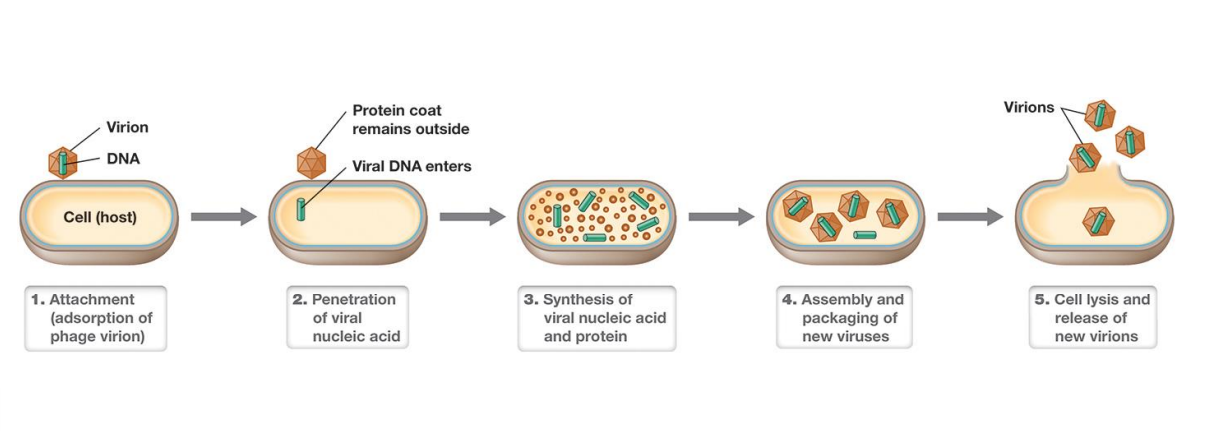
Five steps of viral replication in a permissive (supportive) host?
– attachment (adsorption) of the virion
– penetration (entry, injection) of the virion nucleic acid
– synthesis of virus nucleic acid and protein by host cell as redirected by virus
– assembly of capsids and packaging of viral genomes into new virions
– release of new virions from host cell
*Know steps and in order
The Replication Cycle of a Lytic Bacterial Virus
Virus replication is typically characterized by a
• Virus replication is typically characterized by a one-step growth curve: virion numbers increase when cells burst

One-Step Growth Curve of Virus Replication
• Eclipse phase: genome replicated and proteins translated
• Maturation: packaging of nucleic acids in capsids
• Latent period: eclipse + maturation
• Release: cell lysis, budding, or excretion
– burst size: number of virions released
Which one of these dsDNA?
a. HIV
b. Influenza
c. Chicken pox
d. Ebola
Chicken Pox
Which one of these has no envelope?
a. Measles
b. Influenza
c. Covid
d. Hepatitis A
Hepatitis A
Which of these viruses can cause cancer?
a. HIV
b. Influenza
c. Hepatitis C
d. Ebola
e. HPV
Hepatitis C (liver)
HPV (cervical)
Which of these is an example of a vector transmitted virus?
a. Smallpox
b. Polio
c. Dengue
d. Mumps
e. HPV
Dengue
Which of these viruses is not in Flaviviridae?
a. West Nile
b. Zika
c. Hepatitis C
d. Measles
d. Measles
Why are viruses harder to treat than bacteria?
Viruses are mutating all the time (faster than bacteria)
Virus families
*Polio is an oral vaccine
*Hantavirus now in Hantaviridae used to be in bunyaviridae
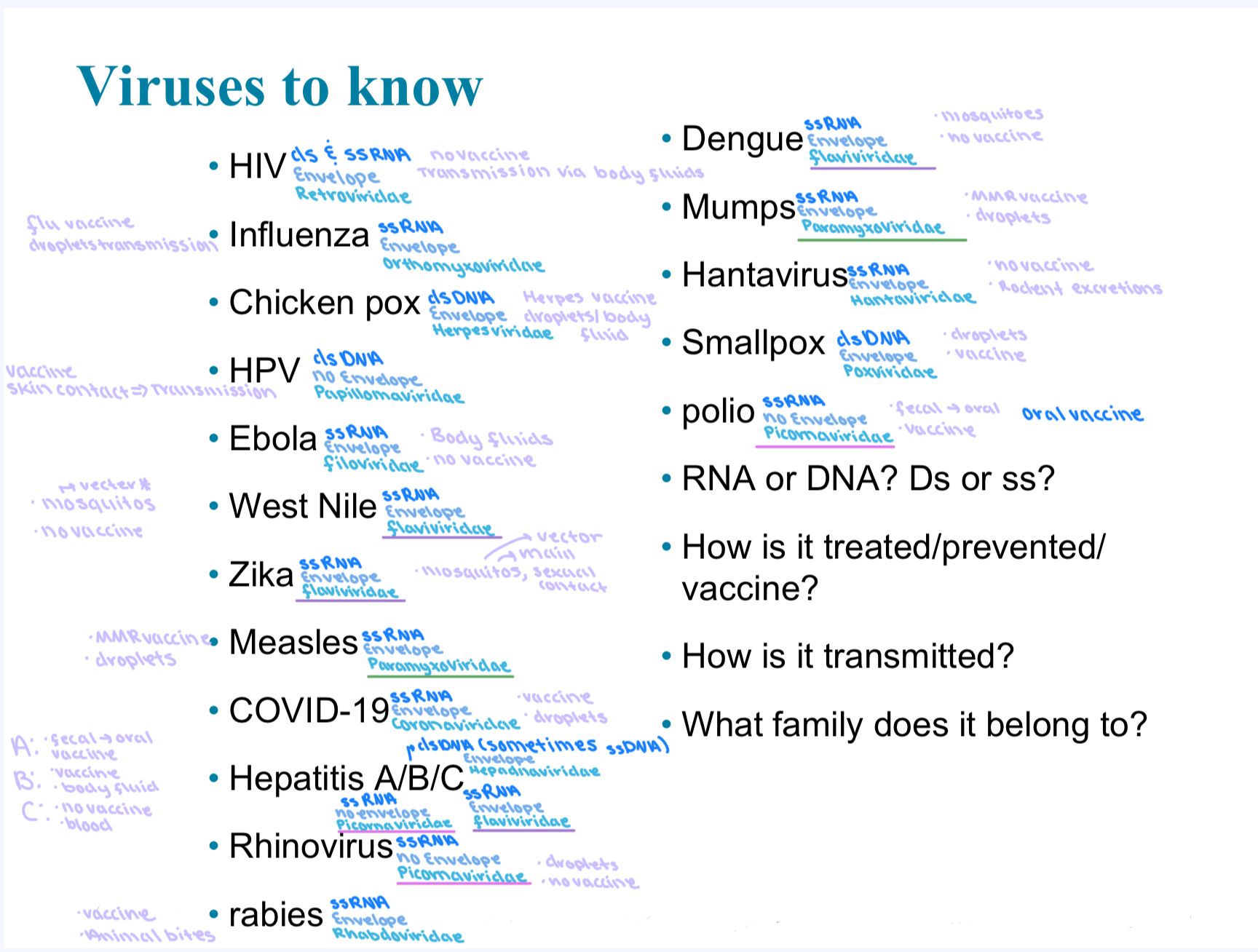
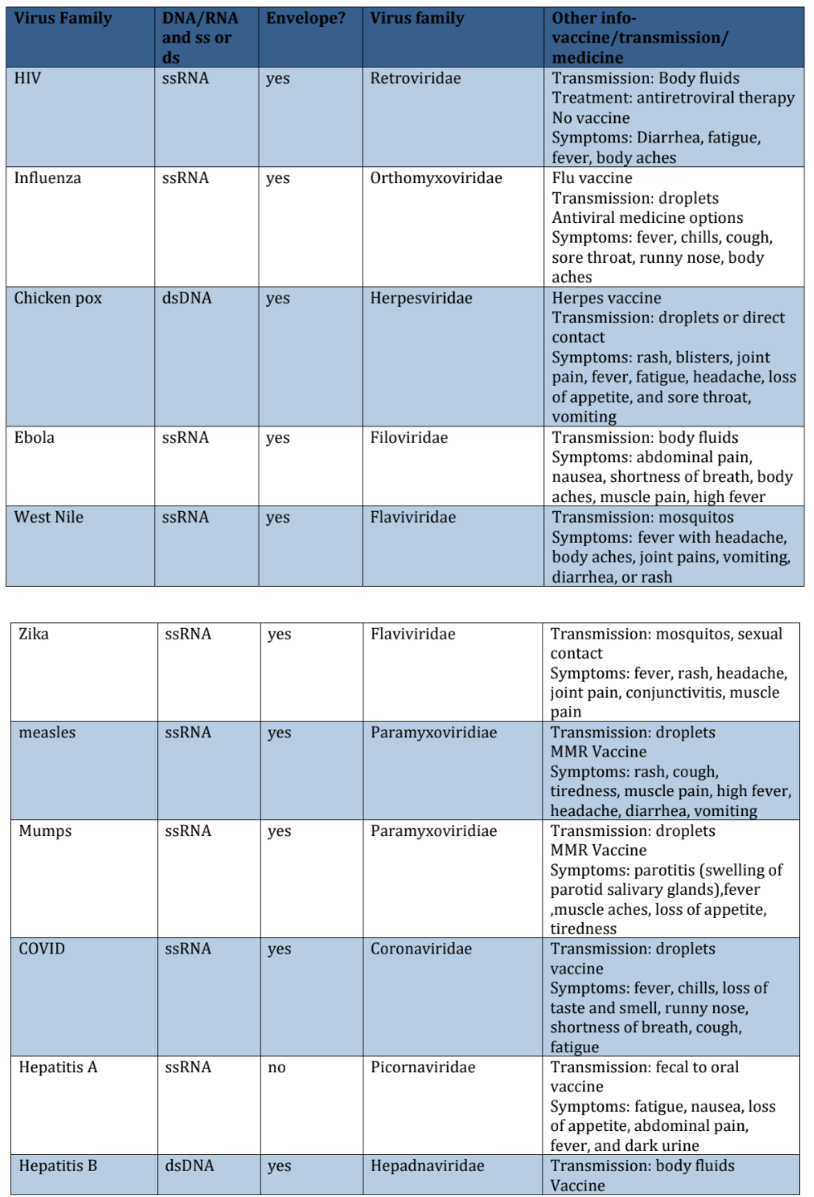
Virus Chart
Hep B are mostly dsDNA but sometimes ssDNA
HIV ds and ssRNA
Which virus has dsDNA and no envelope?
HPV
Which viruses are ssRNA and no envelope?
Hepatitis A
Rhinovirus
Polio
What’s a retrovirus and what is an example of a retrovirus?
A retrovirus is a type of virus that uses RNA as its genetic material instead of DNA. After infecting a cell, it uses an enzyme called reverse transcriptase to convert its RNA into DNA
an example is HIV
Is budding more prevalent in bacterial phages or animal viruses?
animal viruses
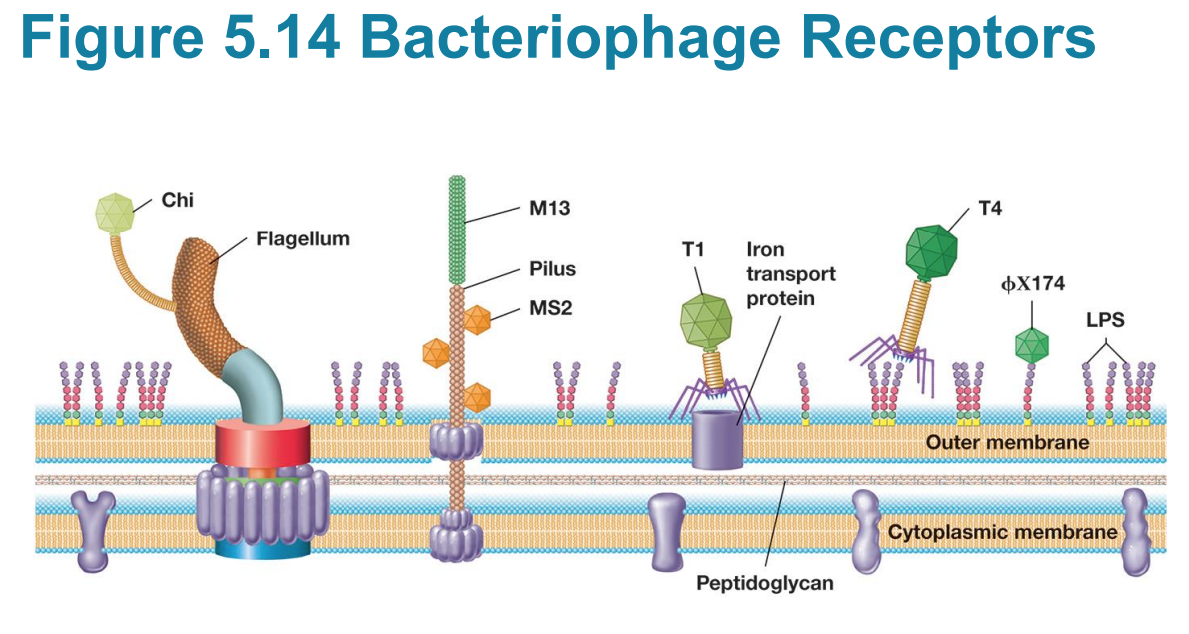
Attachment and Entry of Bacteriophage T4
– major factor in host specificity
Bacteriophage T4 receptors include?
▪ Receptors include proteins, carbohydrates, glycoproteins, lipids, lipoproteins, or other cell structures.
Receptors for T4?
▪ Receptors for T4 are LPS carbohydrate
T4 Lytic Virus Penetration?
– capsid left outside cell
– Viral genome and viral proteins (for some viruses) enter host cell.
▪ Tail fibers retract, and tail pins contact cell wall.
▪ T4 lysozyme forms small pore in peptidoglycan.
▪ Tail sheath contracts, and viral DNA enters cytoplasm similar to syringe injection
▪ Capsid stays outside.
How do bacteria fight back against phages?
▪ toxin- (or) antitoxin molecules (to attack virus)
▪ antiviral CRISPR
▪ restriction endonucleases: enzymes that cleave foreign DNA at specific sites
CRISPR bacterial mechanism that degrades foreign DNA
>adaptive immune system in bac.
~Basis of gene therapy and gene editing
Steps in Lytic Phages
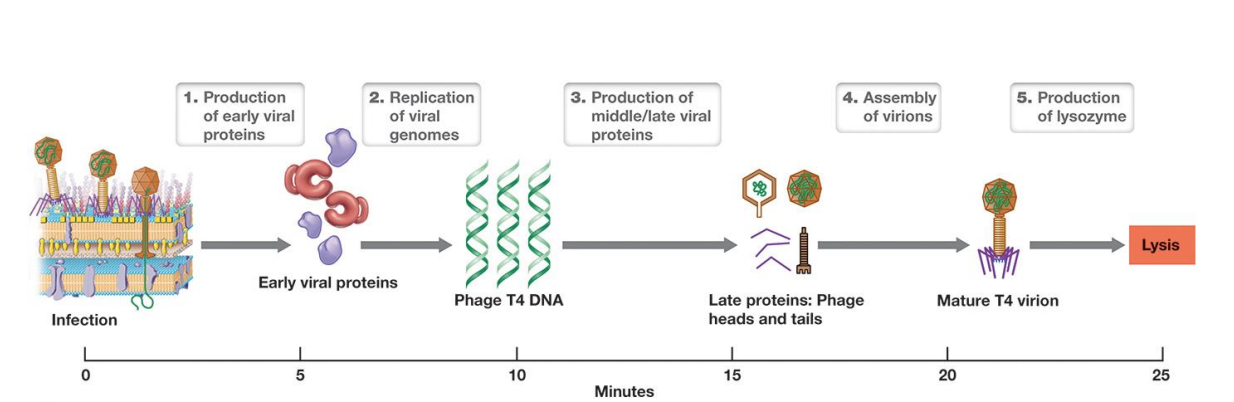
Viral Life Cycles Lysogenic
– Virulent: Viruses always lyse and kill host after infection.
(Go through lysogeny or lysogenic phase)
– Temperate: Viruses establish long-term, stable relationship but are capable of virulence.
▪ can enter lysogeny: few viral proteins produced, viral genome is replicated with host chromosome and passed to daughter cells
▪ lysogen: host cell that harbors temperate virus
Life cycle of a temperate phage
– in lysogeny, genome is either integrated into bacterial chromosome forming prophage (viral DNA)
– Inactivation of repressor induces lytic stage (induction)
▪ Viral DNA excised; phage early, middle, and late proteins produced; virions produced and host lyses
– Cell stress (e.g., DNA damage) induces lytic pathway.
*Induction is the switch to lytic
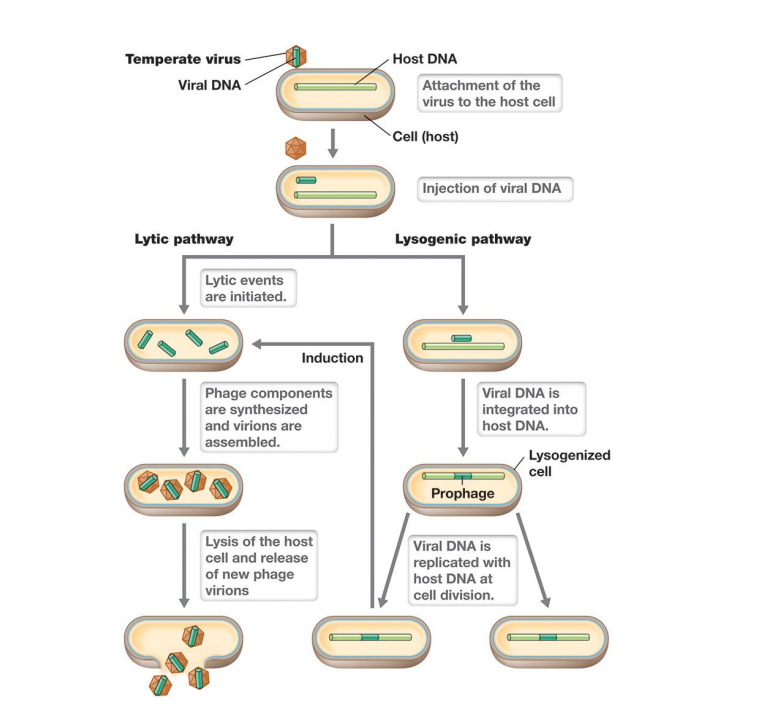
Three key differences
Eukaryotic viruses classified by genomes
– Entire virion enters the animal cell.
– Eukaryotic cells contain a nucleus, the site of replication for many animal viruses.
Viral Infection of Animal Cells
– Studied in cell culture
– Bind specific host cell receptors, typically used for cell-cell contact or immune function (e.g., poliovirus and HIV receptors)
– Viruses often infect only certain tissues because different surface proteins expressed by different tissues/organs
– Host cell entry occurs by fusion with cytoplasmic membrane or endocytosis
– Uncoating occurs at cytoplasmic membrane or cytoplasm.
– Viral DNA genomes enter nucleus, most viral RNA genomes are replicated or converted to DNA within nucleocapsid.
After genome packaging, many animal viruses must be enveloped
Occurs during exit through lysis or budding when virus picks up part of cell’s cytoplasmic membrane and uses it as part of envelope
Animal Virion Assembly and Infection Outcomes
– At least four outcomes
▪ Virulent infection: lysis of host cell, most common
▪ Latent infection: Viral DNA exists in host genome as provirus (similar to lysogeny) and virions are not produced; host cell is unharmed unless/until virulent pathway is triggered.
▪ Persistent infections: slow release of virions from host cell by budding does not result in cell lysis. – Infected cell remains alive and continues to produce virus
▪ Transformation: conversion of normal cell into tumor cell

Vectors
pests that transfer viruses to other host cell types
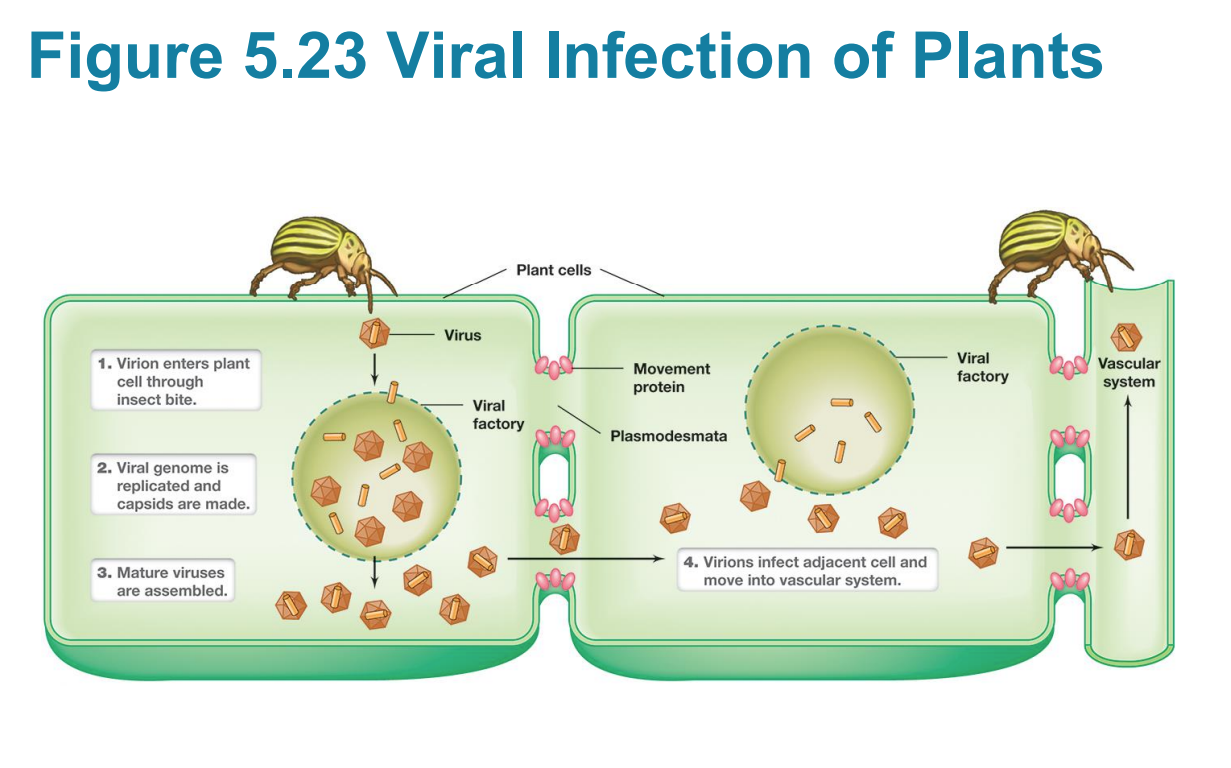
Covid Antigenic Variation Article
Antigenic drift substantially limits the duration of immunity conferred by infection and vaccination by reducing the efficacy of the existing immune effectors that target the drifted region of the viral protein.
Genetic drift is the result of viral mutation rates (change in allele frequency because of mutation) on the other hand antigenic drift is a result of a specific scenario where mutations cause an amino acid substitution which lowers the antigenicity of an immune target the consequential phenotypic result is antigenic drift.
Antigenic drift compromises immunity in a person vaccinated or having previously recovered from COVID by utilizing viruses that escaped the immune system and allowing them to become dominant and replace the original virus
The evolutionary fitness landscape (fitness changing based on genetics) changing with every mutation coupled with randomness of mutation and selection means it is basically impossible to predict what mutation will occur and create vaccines based on the prediction.
End of ch.5 if you’re going in order
End of ch.5 if you’re going in orde
Race Against Resistance
-Dissapearing efficay of antibiotics
-Phage are the most prolific bacteria killers in the world
-Flemings experiment=> first antibiotic
-MRSA methicillin resistant Staphylococcus aureus
-Pseudomonas can have drug resistant species
History
• Egyptians used moldy bread to treat wounds, use of foxglove plants to treat heart disease, use of caraway seeds to treat cancer in India
• Paul Ehrlich- salvarsan 1910 to treat syphilis
• Alexander Fleming- 1928, observed mold inhibited bacterial growth
• 1930’s began “modern medicine” with use of penicillin and sulfa drugs
• Penicillin wasn’t mass produced until 1940’s
What scientist proposed use of salvarsan in 1910 and what disease was it aimed to treat?
The scientist who proposed the use of Salvarsan in 1910 was Paul Ehrlich, and it was developed to treat syphilis.
Antibiotics are antimicrobials naturally produced
• Antibiotics are antimicrobials naturally produced by microbes
– kill or inhibit bacterial growth
– target essential molecular processes
Antimicrobial agent
- groups of chemotherapeutics used to treat diseases caused by microbes
Antibiotic
“against life”, inhibits growth or even destroys the microbe, term originally used for those chemicals produced naturally
What genus of bacteria did the woman with cystic fibrosis that had antibiotic resistance and what therapy ultimately was successful in treating her?
Pseudomonas (aeruginosa)
Phage therapy
Synthetic
made in lab
Spectrum of activity
range of different microbes against which an agent acts
• broad vs narrow
Modes of action
“Target”
- inhibit cell wall synthesis, disrupt cell membrane function, inhibit protein synthesis, inhibit nucleic acid synthesis, act as an antimetabolite
Many antibiotics target
– Many antibiotics target DNA replication, RNA synthesis, and translation
▪ Quinolones target DNA gyrase and topoisomerase IV by interfering with DNA unwinding and replication
▪ Rifampin and actinomycin prevent RNA synthesis (transcription) by blocking RNA polymerase active site or RNA elongation
– Inhibition of protein synthesis
▪ Aminoglycoside antibiotics (e.g., streptomycin) target 16S rRNA of 30S ribosome (inhibits protein synthesis) , leading to error-filled proteins that inhibit growth
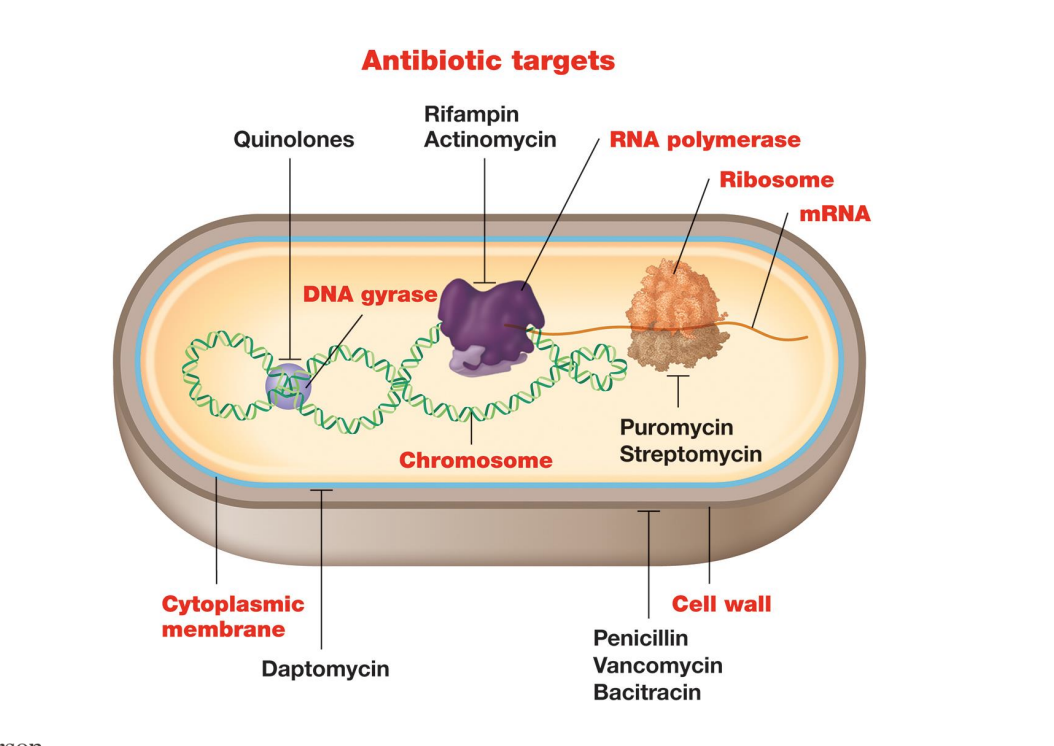
Polymyxins are cyclic peptides whose long hydrophobic tails target the what?
– Polymyxins are cyclic peptides whose long hydrophobic tails target LPS layer, disrupting membrane and causing leakage and death
(Gram (-) Ex: E.coli, Pseudomonas
β-lactams (penicillin, cephalosporin, derivatives) interfere with
▪ β-lactams (penicillin, cephalosporin, derivatives) interfere with transpeptidation (formation of crosslinks) between muramic acid residues (cell wall synthesis)
1) Vancomycin binds to what?
2) Bacitracin binds to bactoprenol and prevents new what?
1) Vancomycin binds to pentapeptide precursor and prevents interbridge formation
2) Bacitracin binds to bactoprenol and prevents new peptidoglycan precursors from reaching site of synthesis
Side effects of drugs
• Toxicity (how much of the drug is safe)
• Allergy
• Disruption of normal flora- leading to secondary infections
Resistance mechanisms
– Resistance mechanisms genetically encoded in four classes: 1)modification of drug target, 2)enzymatic inactivation, 3)removal via efflux pumps, 4)metabolic bypasses ( how bacteria fight back)
– Random chromosomal mutations can lead to resistance
– Resistance genes can exist on mobile genetic elements and be transferred by horizontal gene flow
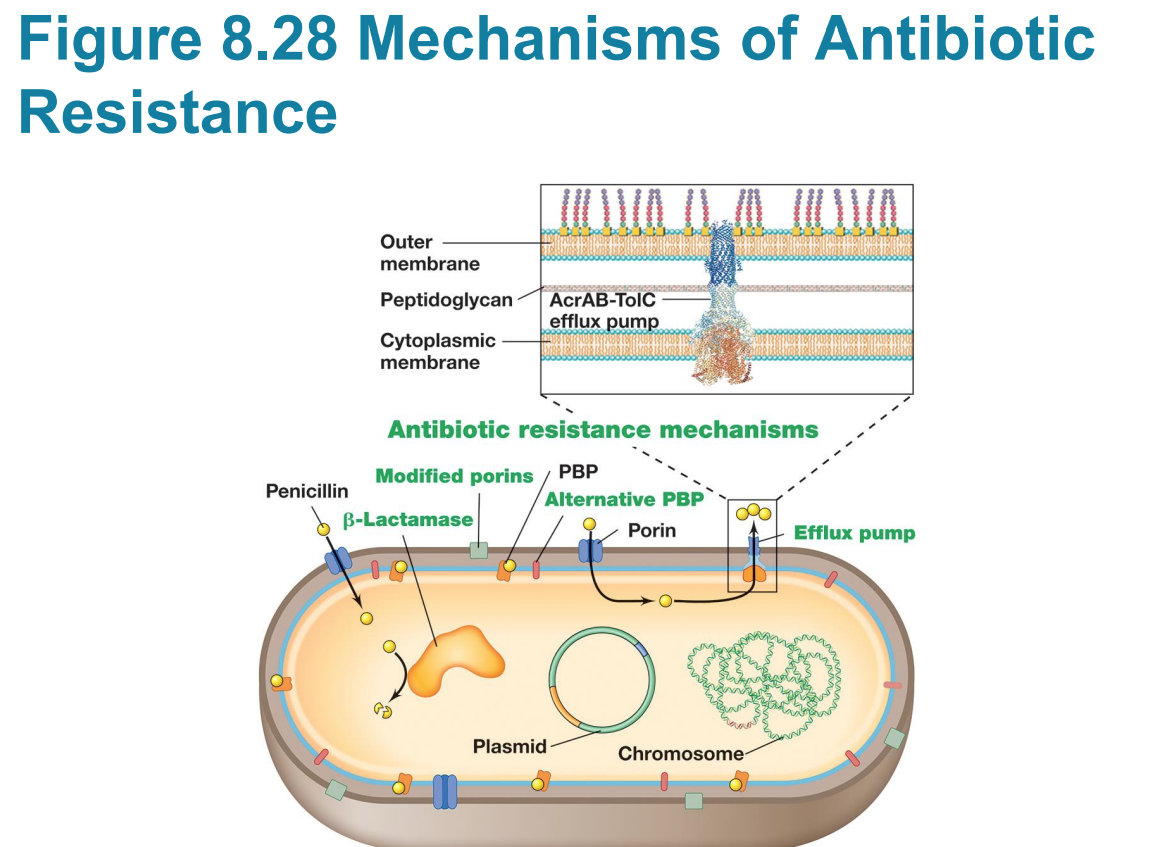
Efflux pump
– Efflux pumps are ubiquitous and transport various molecules, including antibiotics, out of the cell
▪ Lowers intracellular concentration, allowing cell to survive at higher external concentrations
▪ Many act promiscuously and transport different antibiotic classes, contributing to multidrug resistance
▪ E. coli has one of the best characterized; pumps out rifampicin, chloramphenicol, fluoroquinolones
question: What is an efflux pump?
An efflux pump is a protein transporter in cell membranes that expels molecules from a cell, playing a major role in antibiotic resistance by pumping drugs out before they can be effective
Biofilm growth leads to increased
– Biofilm growth leads to increased resitsance
What is an example of a bacteria with an efflux pump?
▪ Pseudomonas aeruginosa encodes several multidrug efflux pumps that are more active when cells grow in an attached state
Antibiotic target is no longer essential ,example:
– Antibiotic target is no longer essential
▪ example: Methicillin-resistant Staphylococcus aureus (MRSA)
the original antibiotic target (the normal penicillin-binding protein in this case ) is no longer essential for survival, or the target is bypassed by a modified protein that is resistant to the drug.
prevention
• Take full course of antibiotic
• Do not share antibiotics
• Do not take same antibiotic repeatedly- finish first course, infection not go away- talk to physician about trying a different antibiotic as resistance may have developed
• Some infections don’t require an antibiotic (viral, allergies etc)
• Next steps- scientists developing phage therapy
Persistence
Persistence: population of antibiotic-sensitive bacteria produces rare cells that are transiently tolerant to multiple antibiotics
Persisters are genetically identical but
• Persisters are genetically identical but dormant (viable but do not grow)
– Dormancy prevents antibiotic from killing cell
Once antibiotic exposure ends
▪ Once antibiotic exposure ends, persisters exit stringent response pathway and produce antitoxin, returning protein synthesis to normal and allowing cells to grow
End of ch.8 if going in order
End of ch.8 if going in order
Bacterial and archaeal genetics and
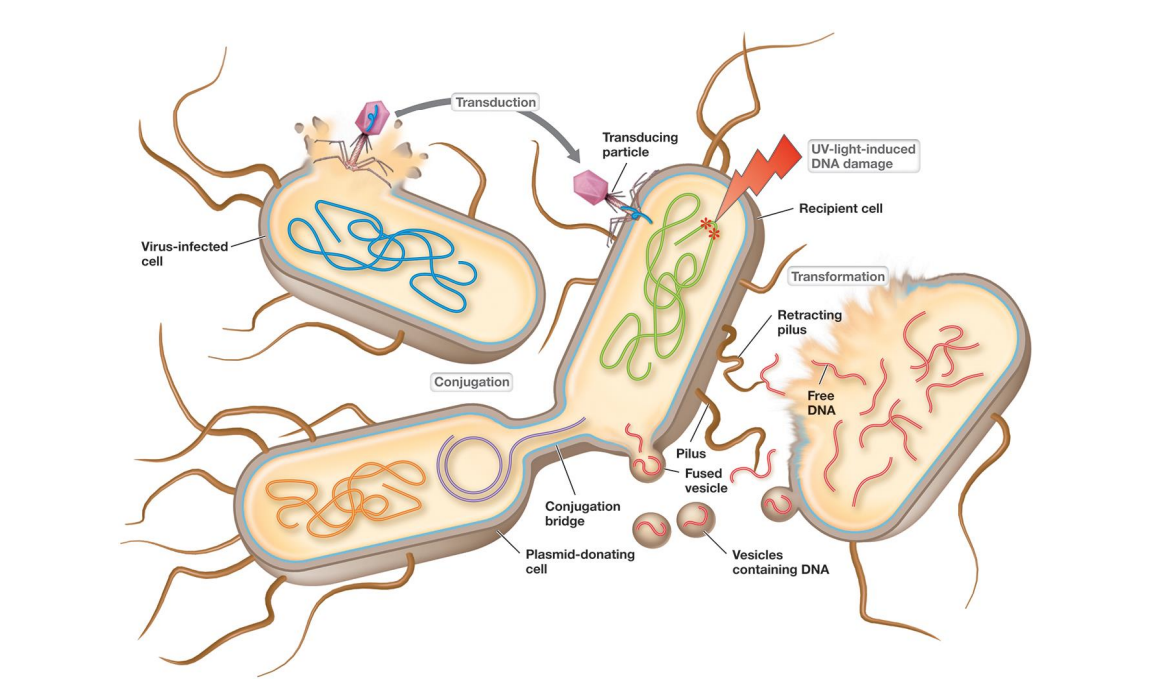
Bacterial and archaeal genetics and horizontal gene transfer (movement of genes between cells other than reproduction)
• Mutation: heritable change in genome – can lead to a change in properties of an organism – some beneficial, some detrimental, most have no effect
Horizontal gene transfer (genetic exchange) generates
Horizontal gene transfer (genetic exchange) generates much larger changes
-gene movement between cells that are not direct descendants
Which of the following genetic transfer methods use a virus?
Transformation
Transduction
Conjugation
Transduction
What is Wild-type strain?
Wild-type strain: isolated from nature (standard)
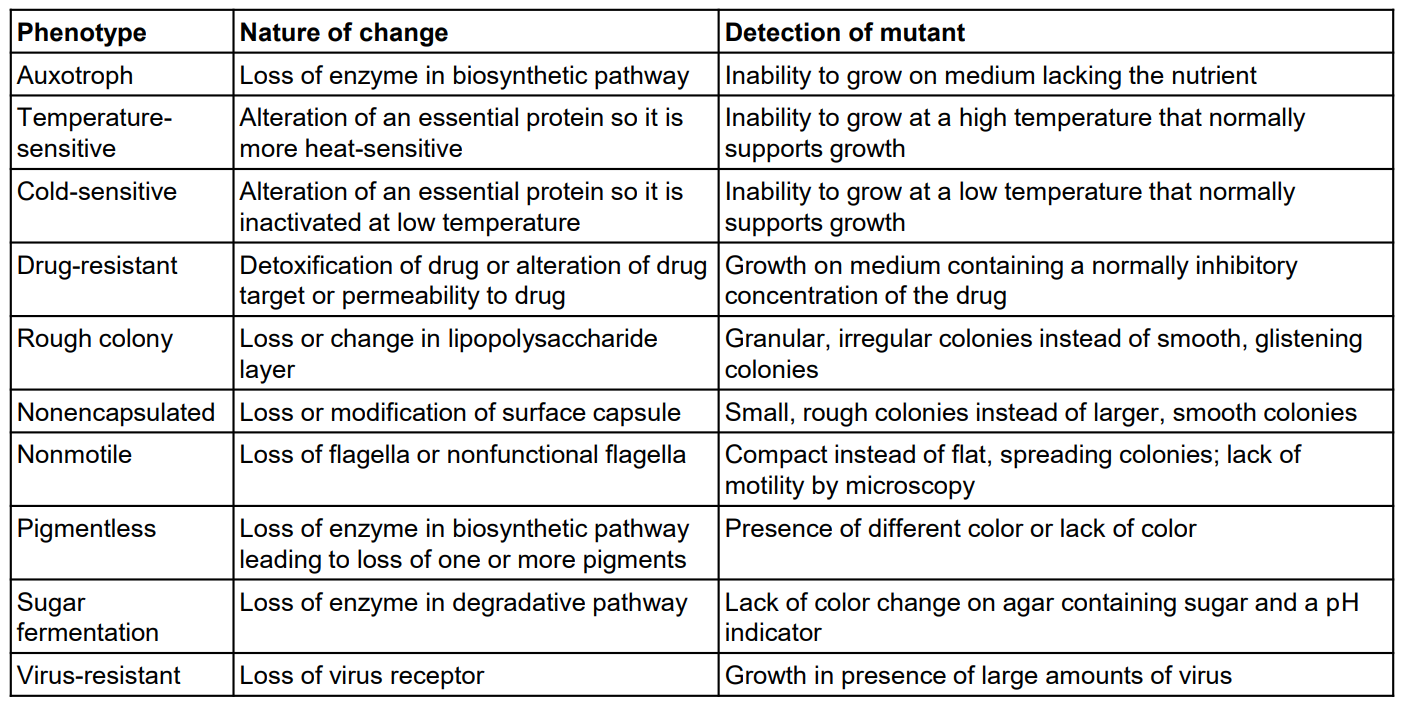
Mutant
• Mutant
– A cell or virus derived from wild type that carries a nucleotide sequence (genotype) change
– Observable properties (phenotype) may also be altered
Selectable mutations
– Selectable mutations confer an advantage
▪ Under certain environmental conditions, progeny cells outgrow and replace parent
▪ Example: antibiotic resistance
▪ Relatively easy to detect
▪ Powerful genetic tool
Besides antibiotic resistance what other mutations do people study?
*Know ex’s
m.c. or S.A
Spontaneous mutations
– Occur without external intervention
– Most result from occasional errors by DNA polymerase during replication
Induced mutations
– Caused environmentally or deliberately
– Can result from exposure to natural radiation or chemicals that chemically modify DNA
Point mutations
– Change only one base pair
– Occurs via single base-pair substitution
– Phenotypic change depends on exact location
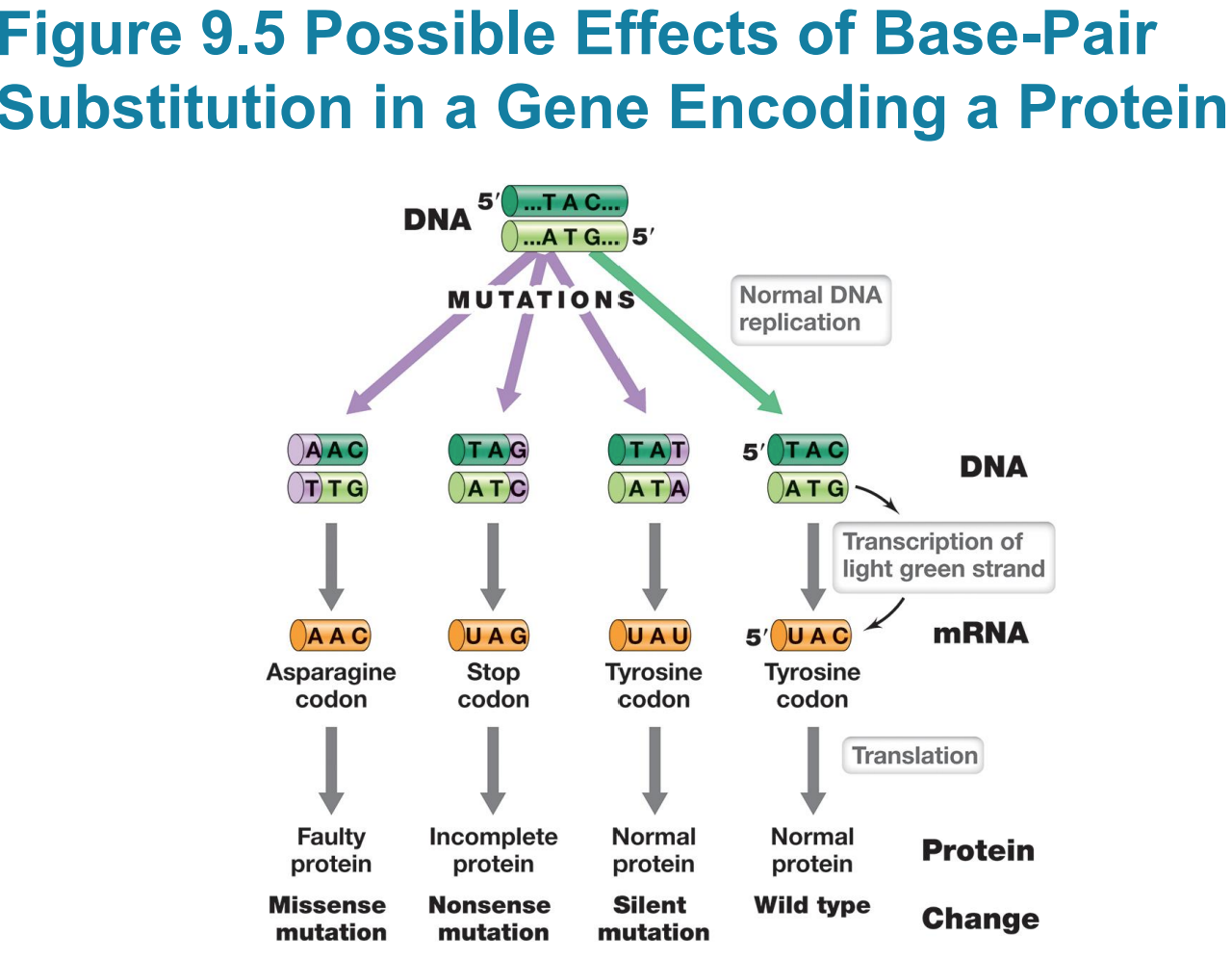
Silent mutations do not affect
– Silent mutations do not affect sequence of encoded polypeptide or phenotype (e.g., UAC to UAU)
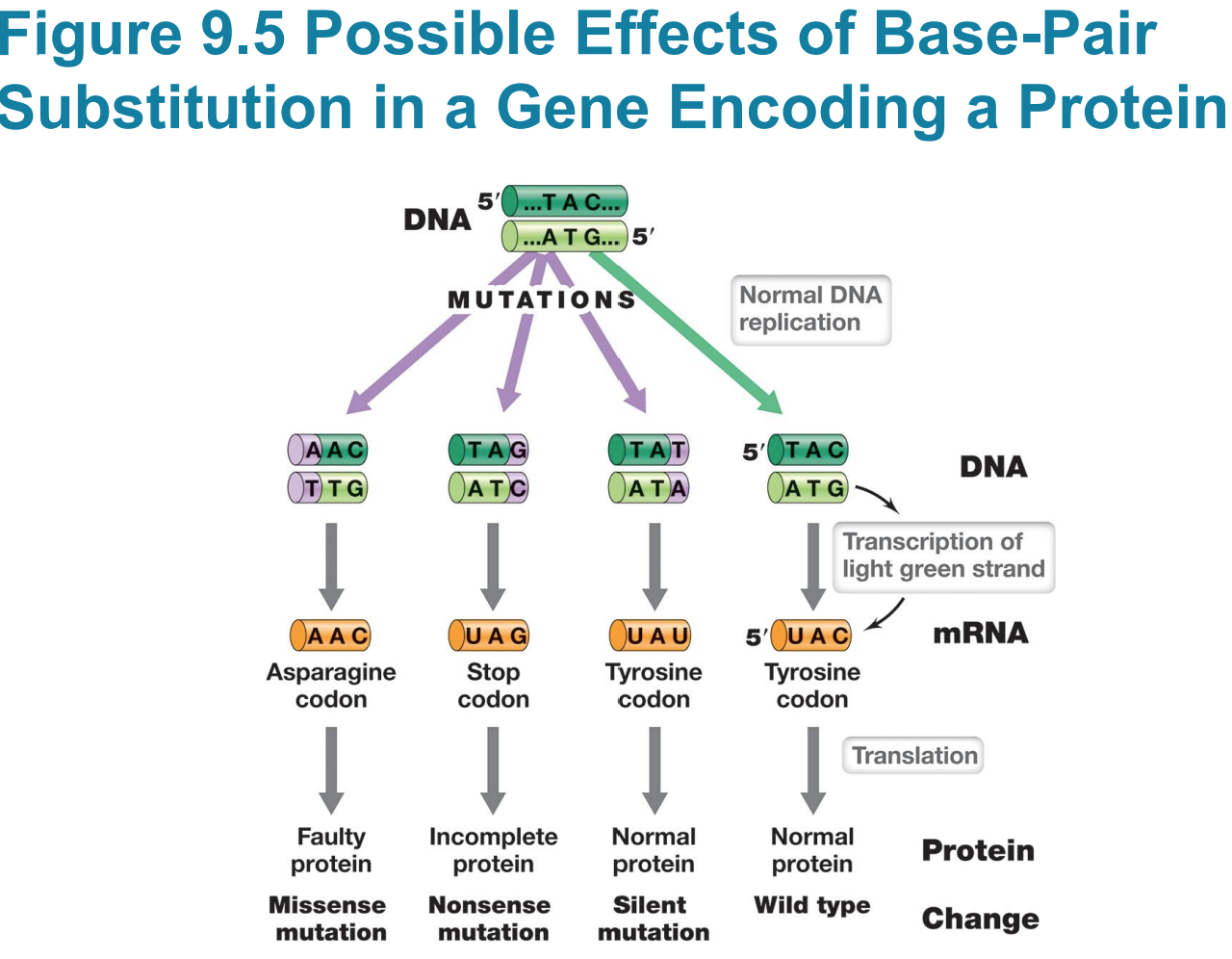
Missense mutation changes sequence
– Missense mutation changes sequence of amino acids in polypeptide (e.g., UAC to AAC)
▪ If at a critical location, e.g., active site, could alter activity
▪ Not all missense mutations lead to dysfunction/nonfunction
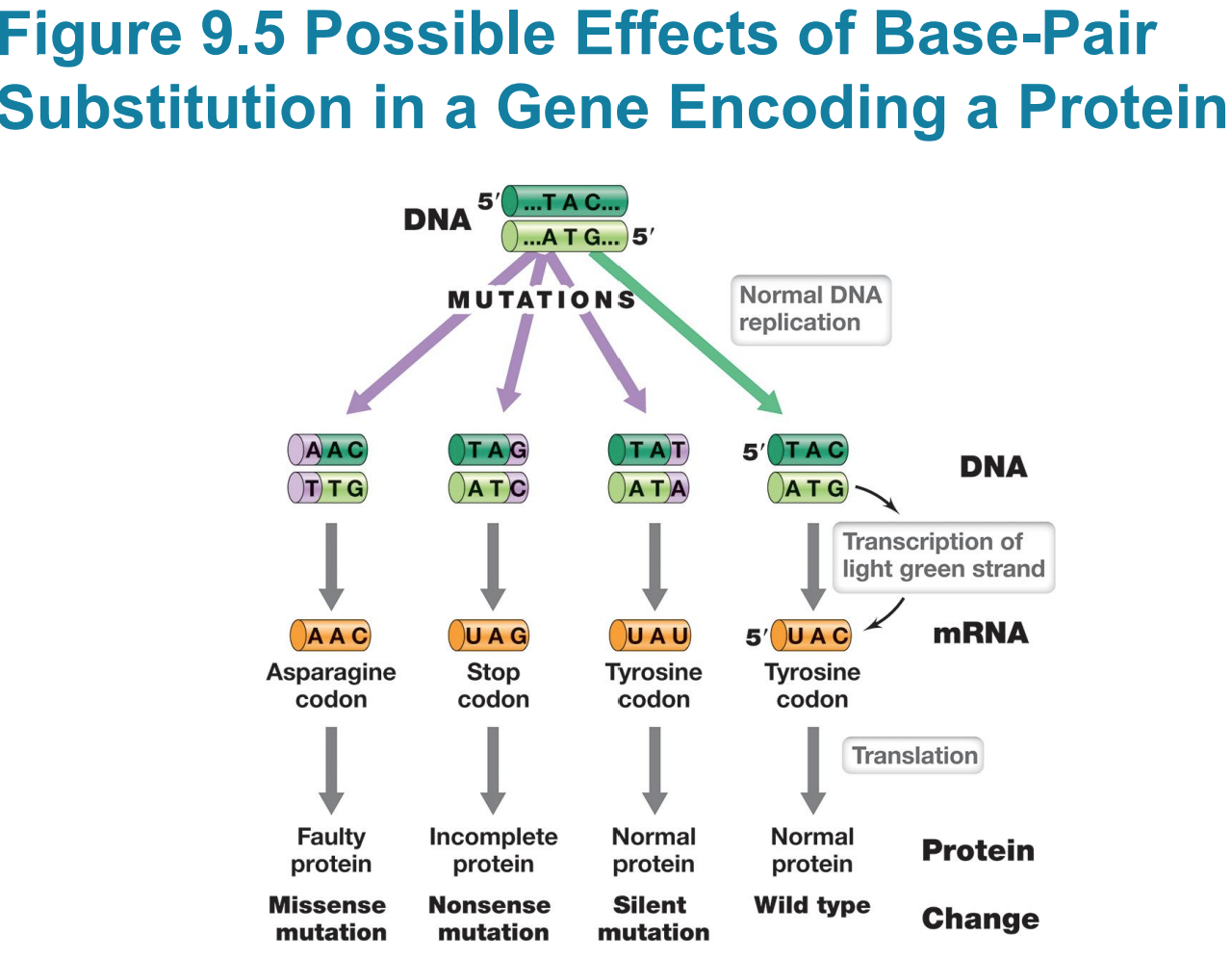
Nonsense mutation (stop codon)
▪ Typically results in truncated (incomplete) protein that lacks normal activity
Transitions vs transversions
– Transitions are purines (A/G) substituted for other purines or pyrimidines (C/T) substituted for other pyrimidines
– Transversions are purines substituted for pyrimidines or vice versa
Frameshift mutations
– Frameshift mutations: single base pair deletions or insertions that result in a shift in the reading frame
▪ Scrambles entire polypeptide sequence downstream
▪ Insertion or deletion of two base pairs also causes frameshift
▪ Insertion/deletion of three base pairs adds/deletes a codon/an amino acid, which usually is not as bad
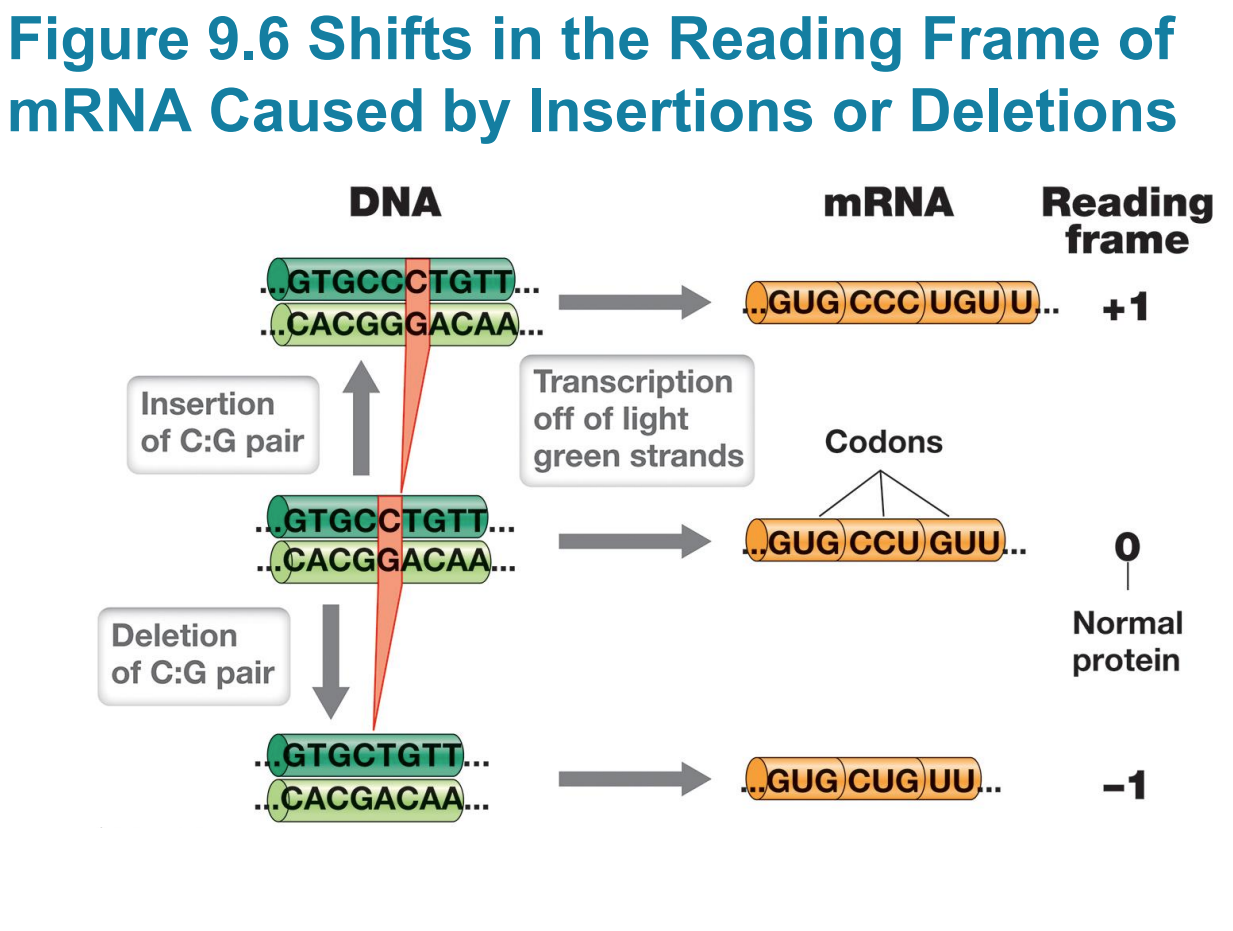
Large insertions may be due to
▪ Large insertions may be due to transposable elements
Eukaryotes have ____lower error rates?
– Eukaryotes have 10-fold lower error rates
Mutation Rates
– Single base errors more likely lead to missense than nonsense mutations because most substitutions still encode amino acids
– Can increase pool of mutations by treatment with mutagenic agents or under high stress