Types of structure: Elements compounds and mixtures: Chemistry: (9:1)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
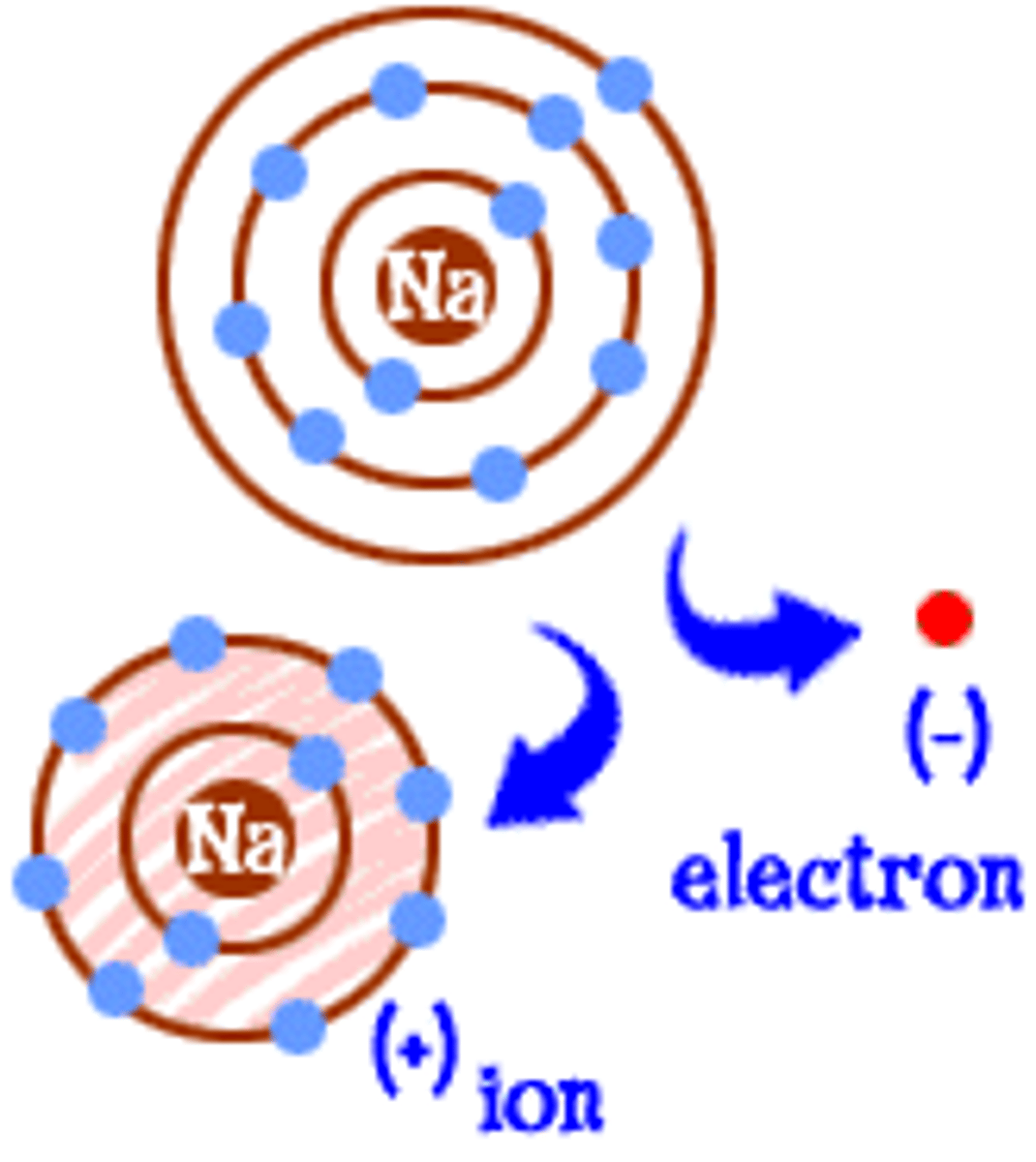
Ion
An atom or group of atoms that has lost or gained electrons and therefore a positive or negative charge.
Polyatomic ion
An ion that is made of more then one atom
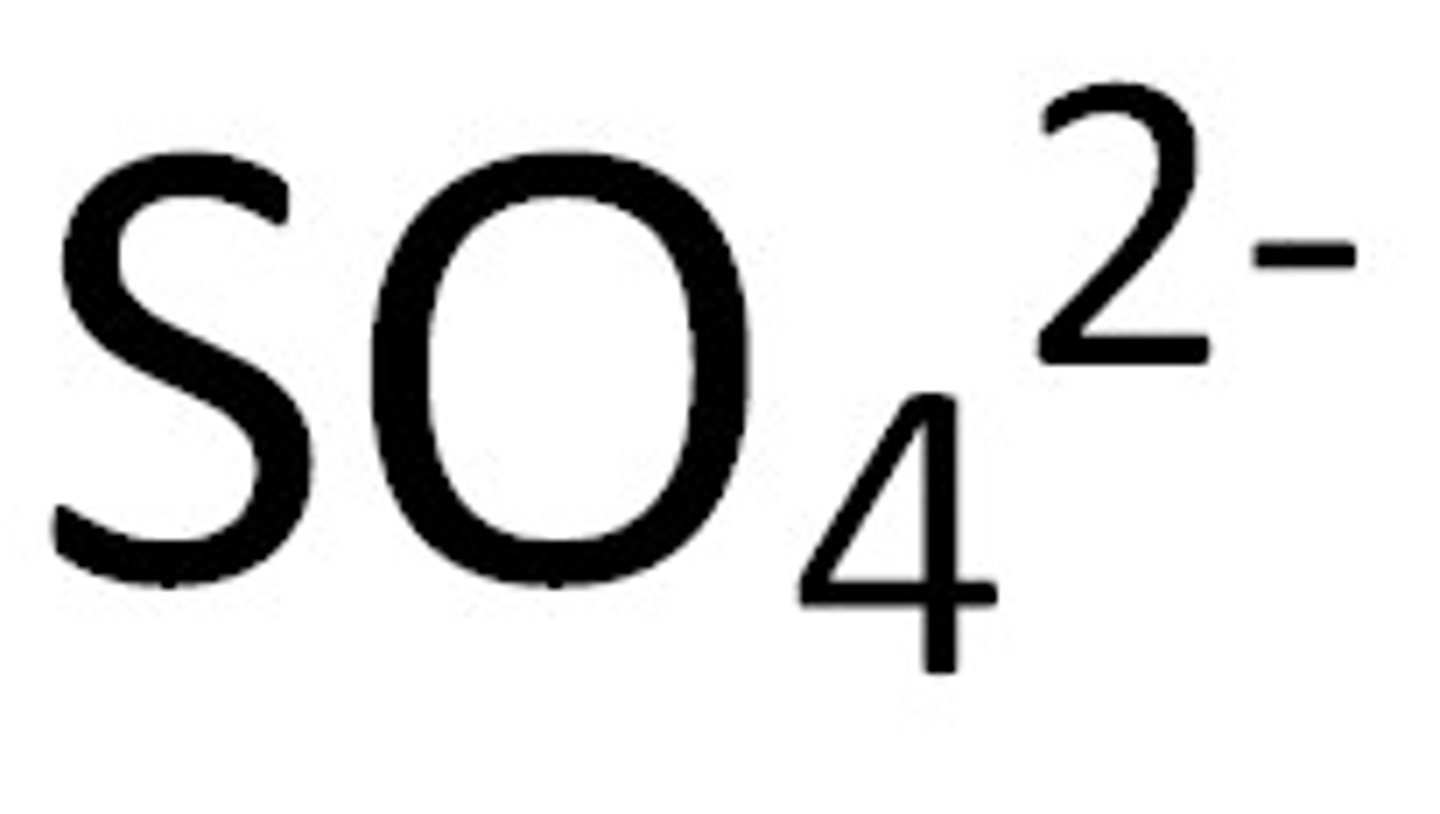
Metal ion
an atom which has lost electrons forming a positive ion
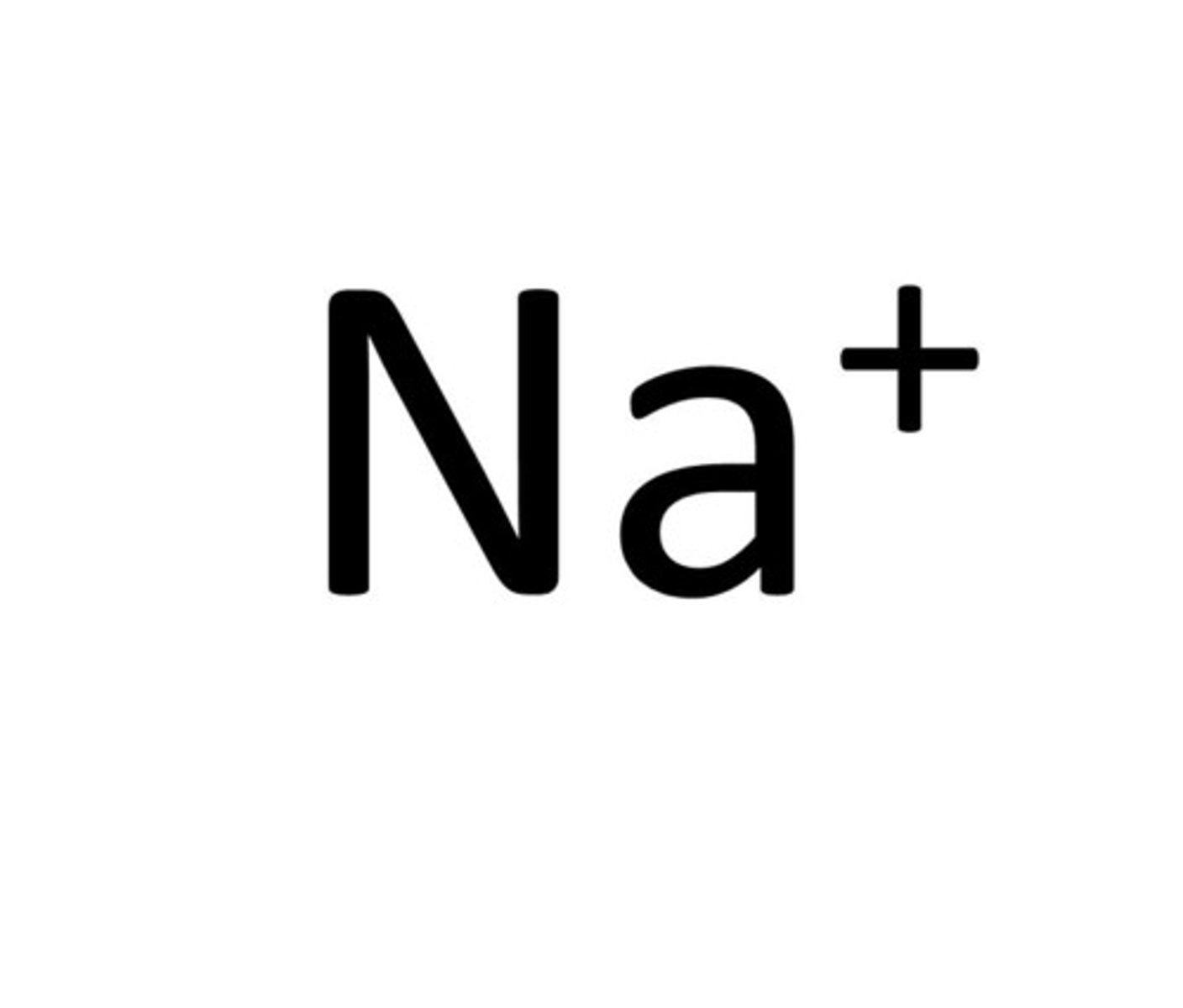
Non-metal ion
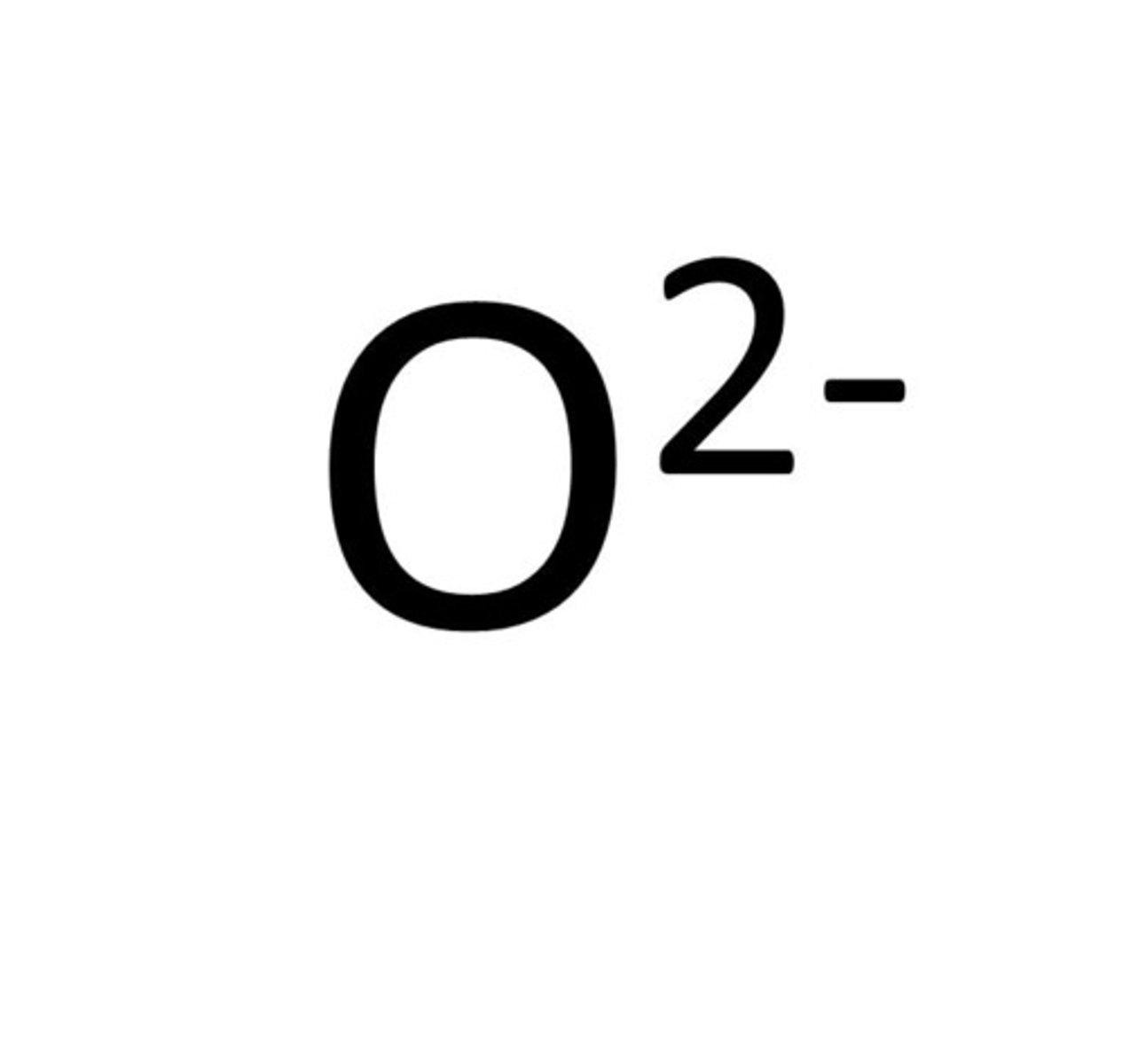
an atom which has gained electrons forming negative ions.
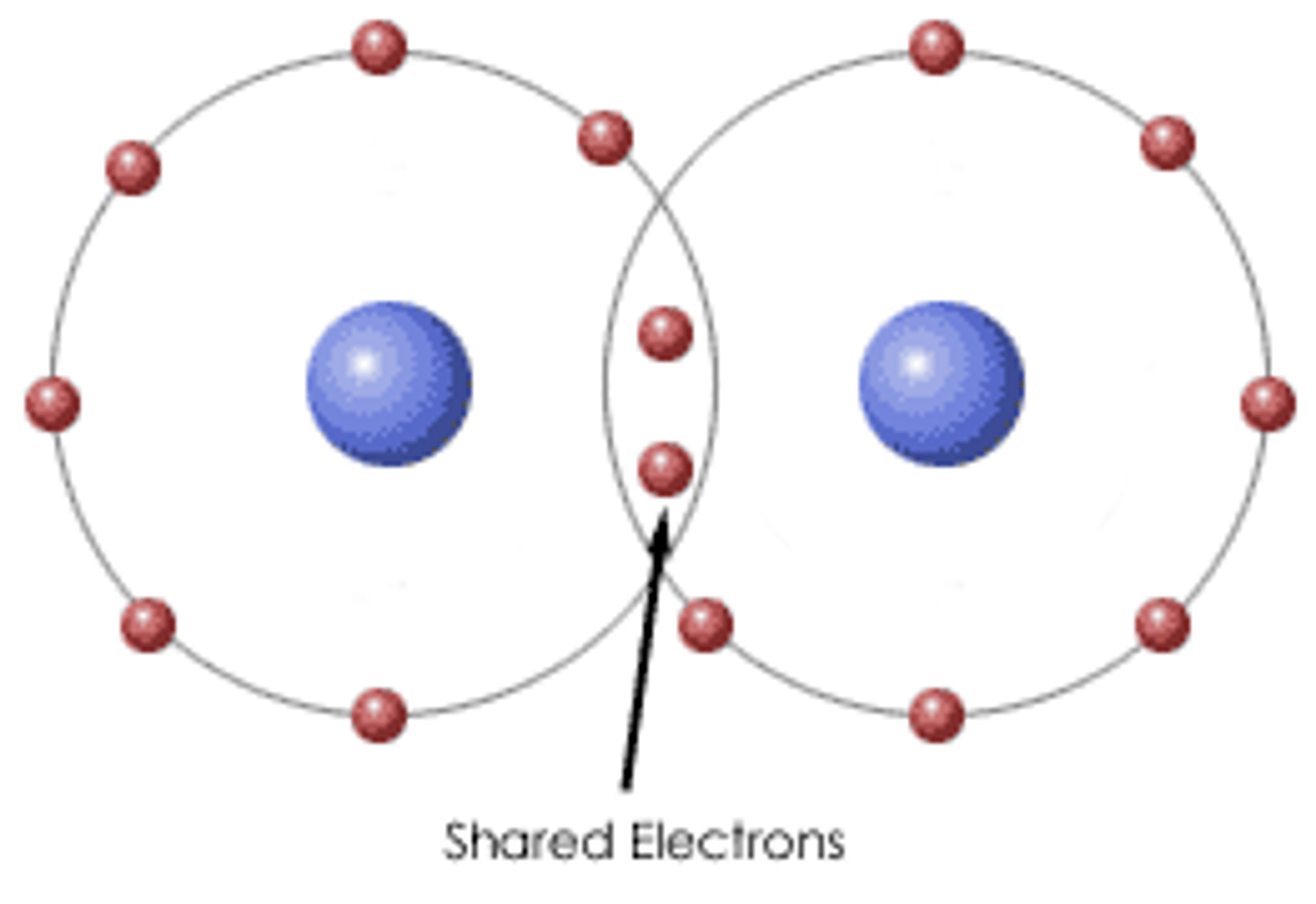
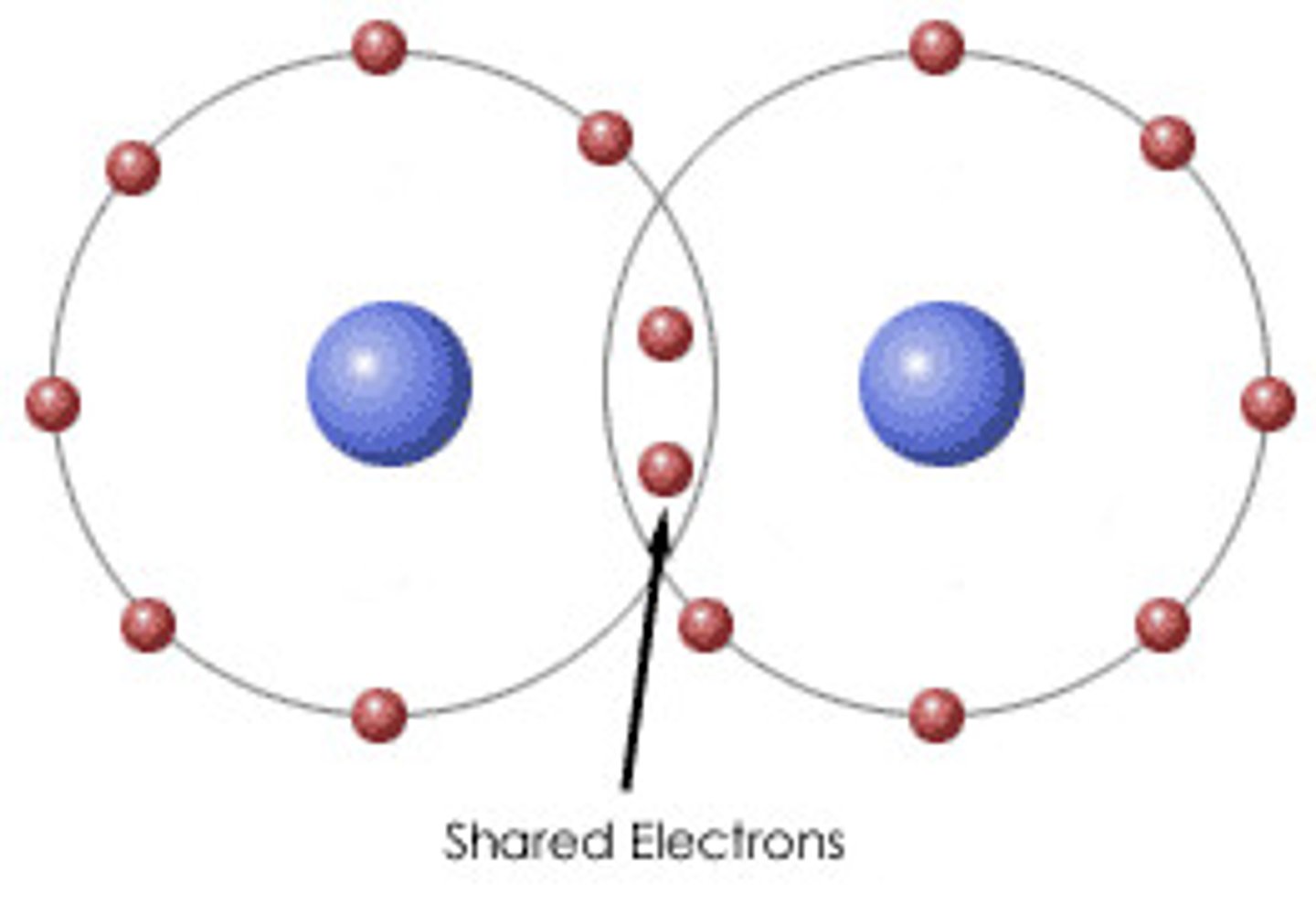
Covalent bond
a shared pair of electrons
Molecule
two or more atoms held together by covalent bonds
Metallic bonding
Positively charged metal ions are surrounded by delocalised outer electrons
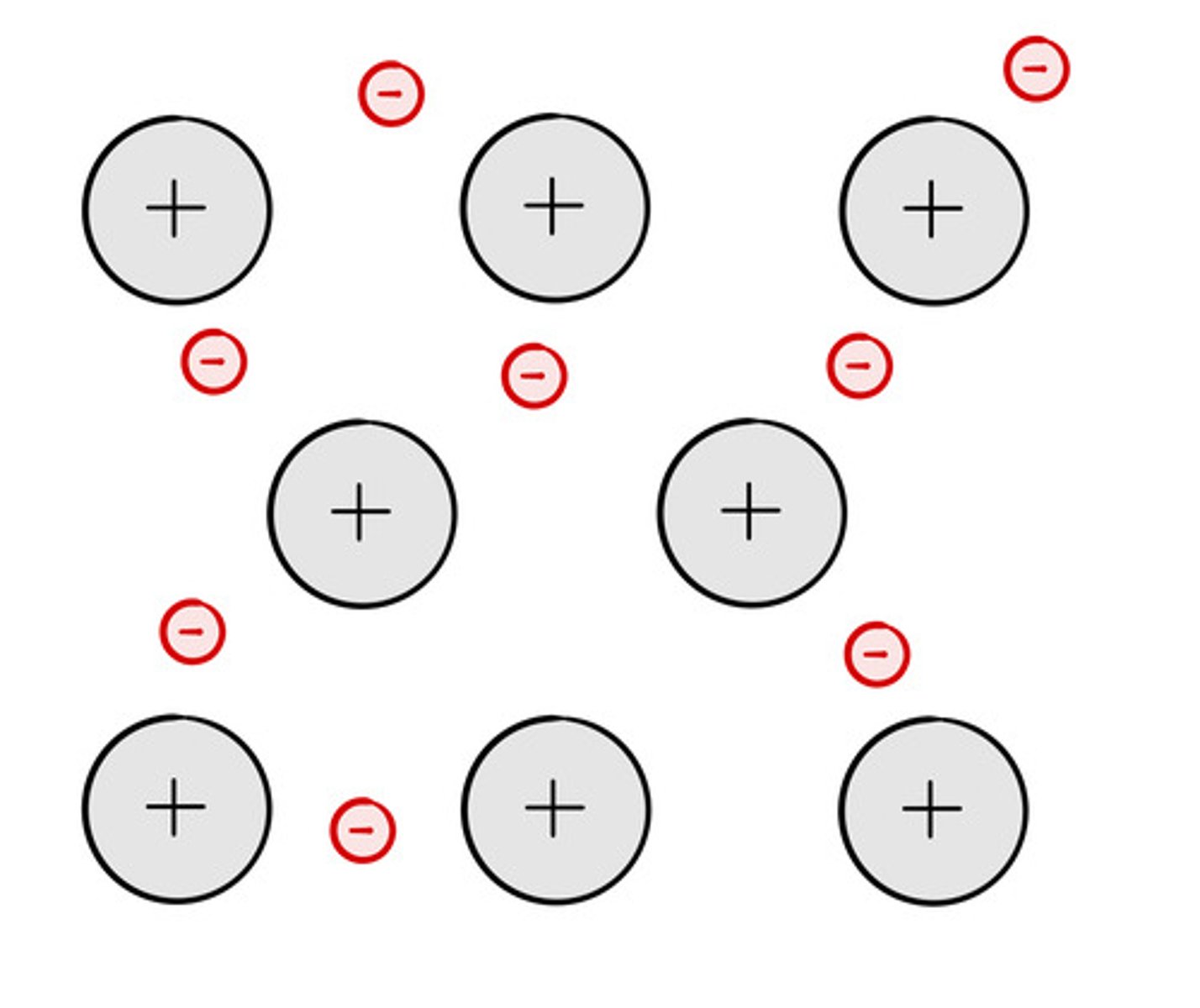
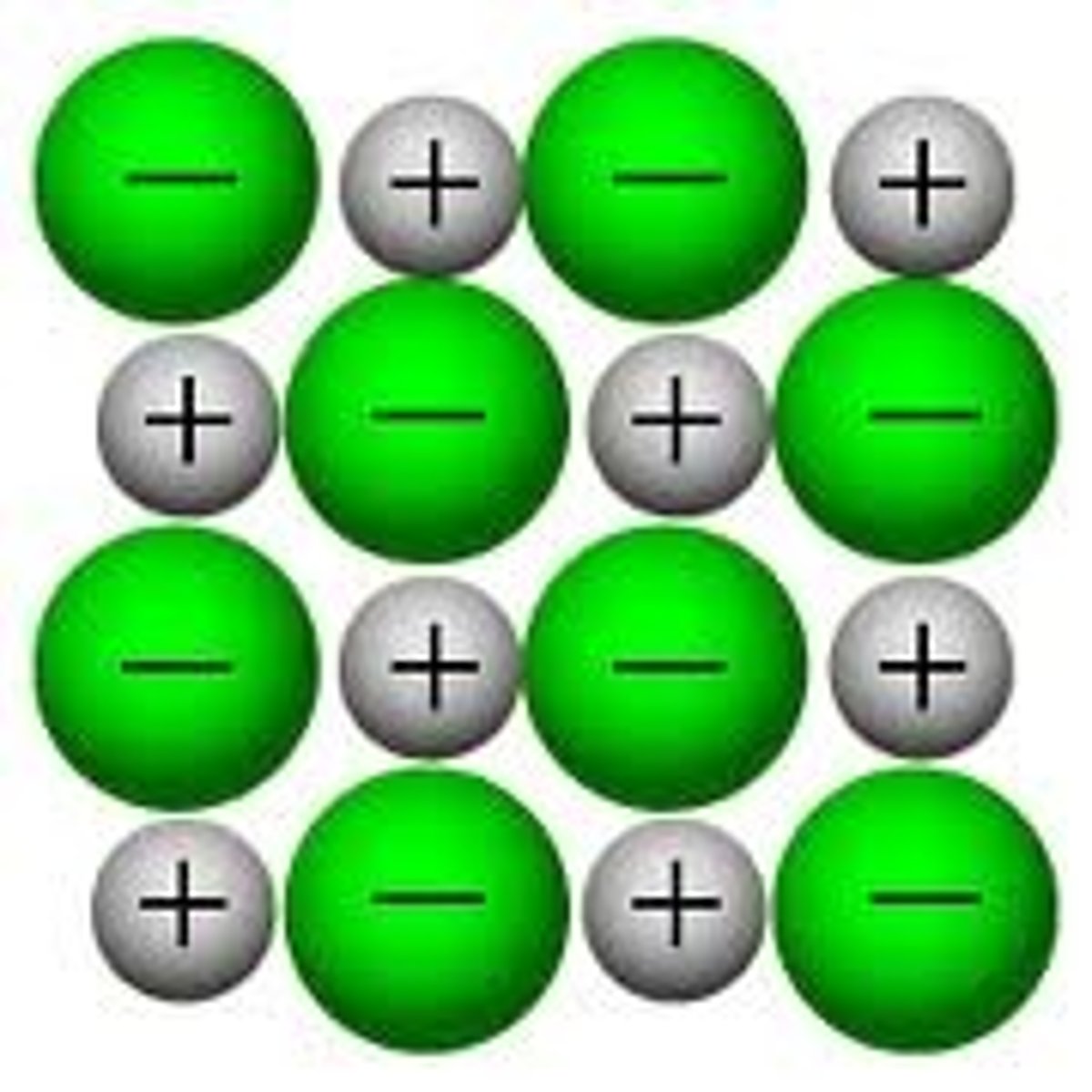
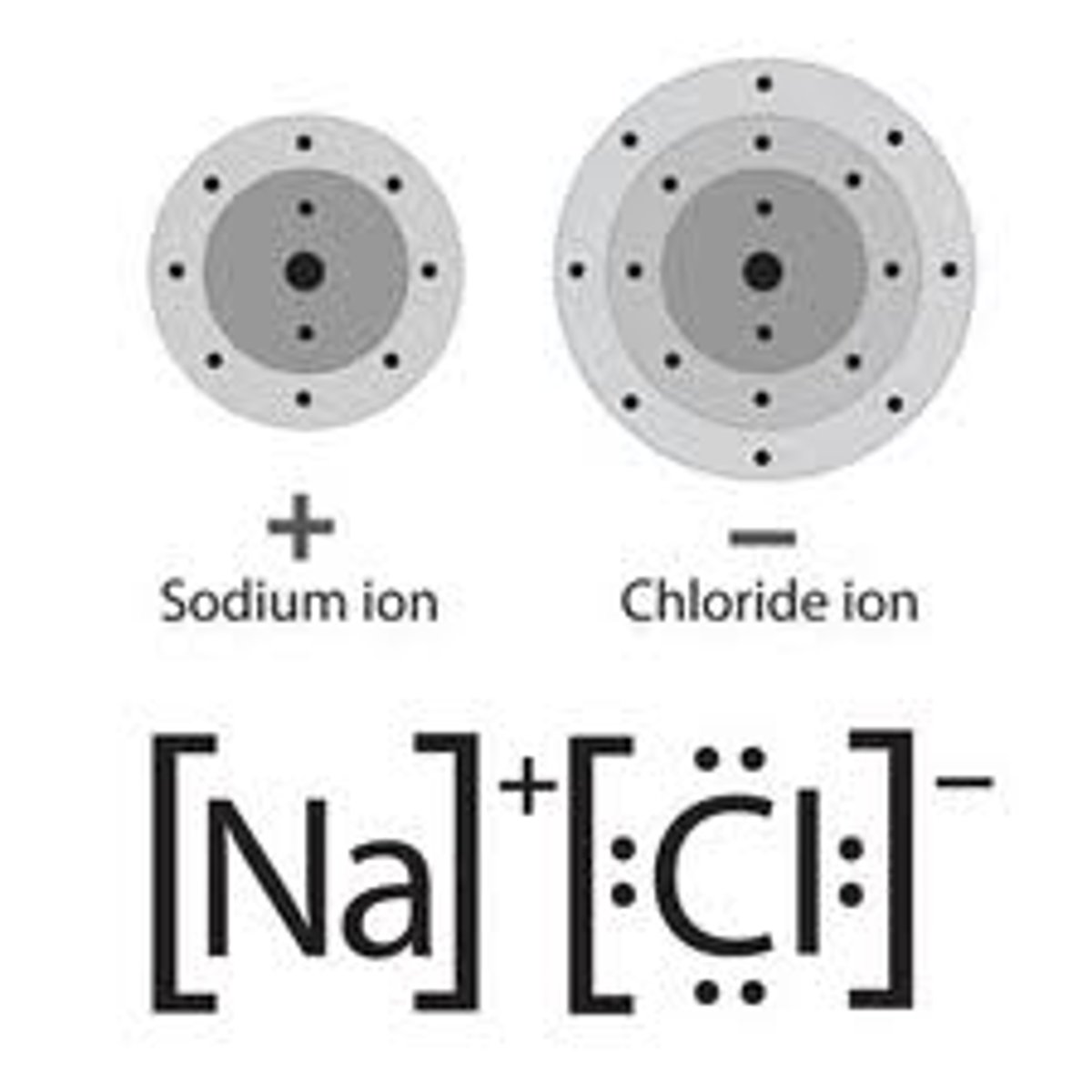
Particles in an ionic compound
Positive and negative ions
Particle in a simple covalent substance
Molecules
Particles in a giant covalent substance
Covalently bonded atoms
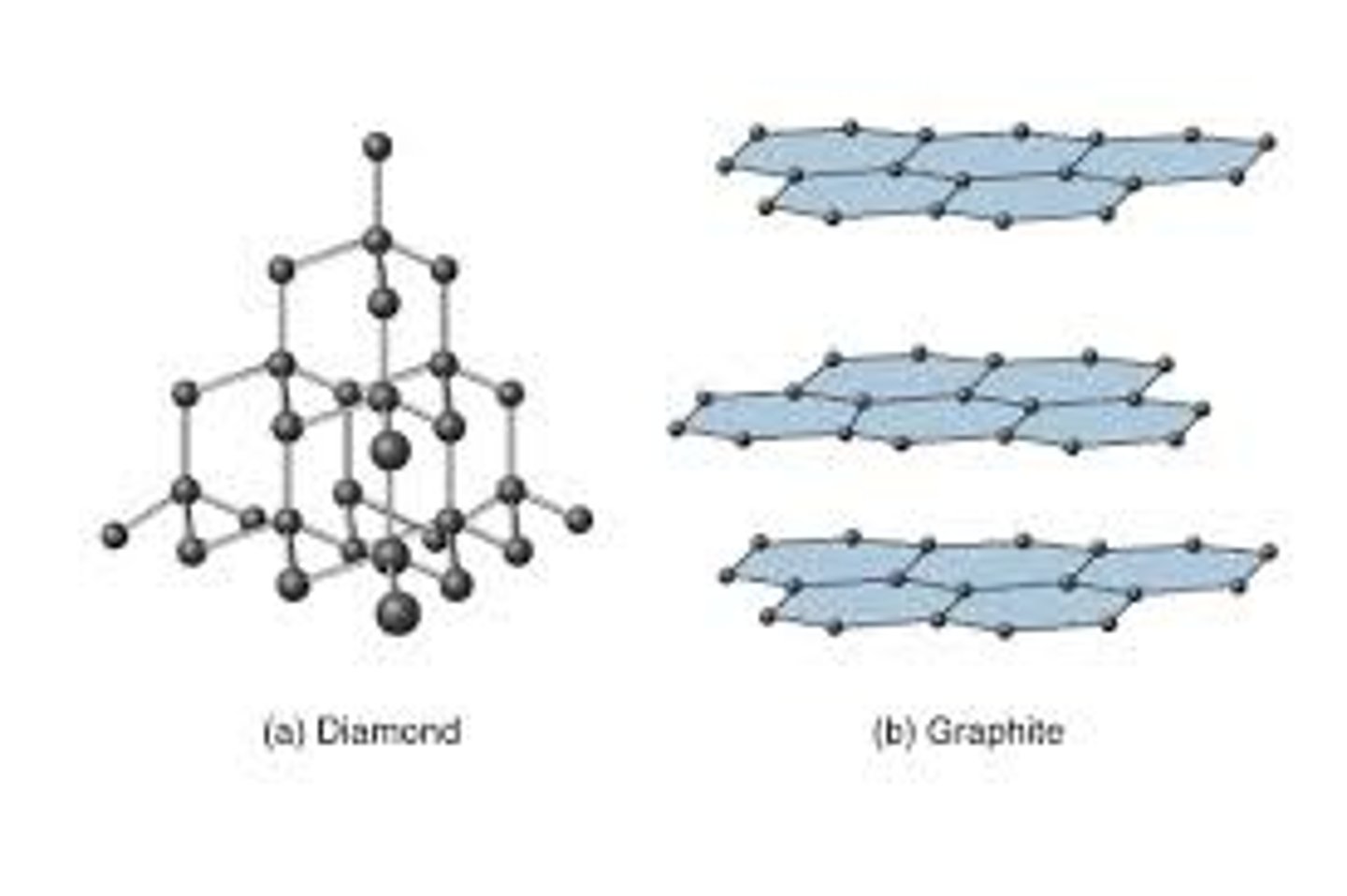
Particles in a metallic substance
Positive ions and delocalised electrons
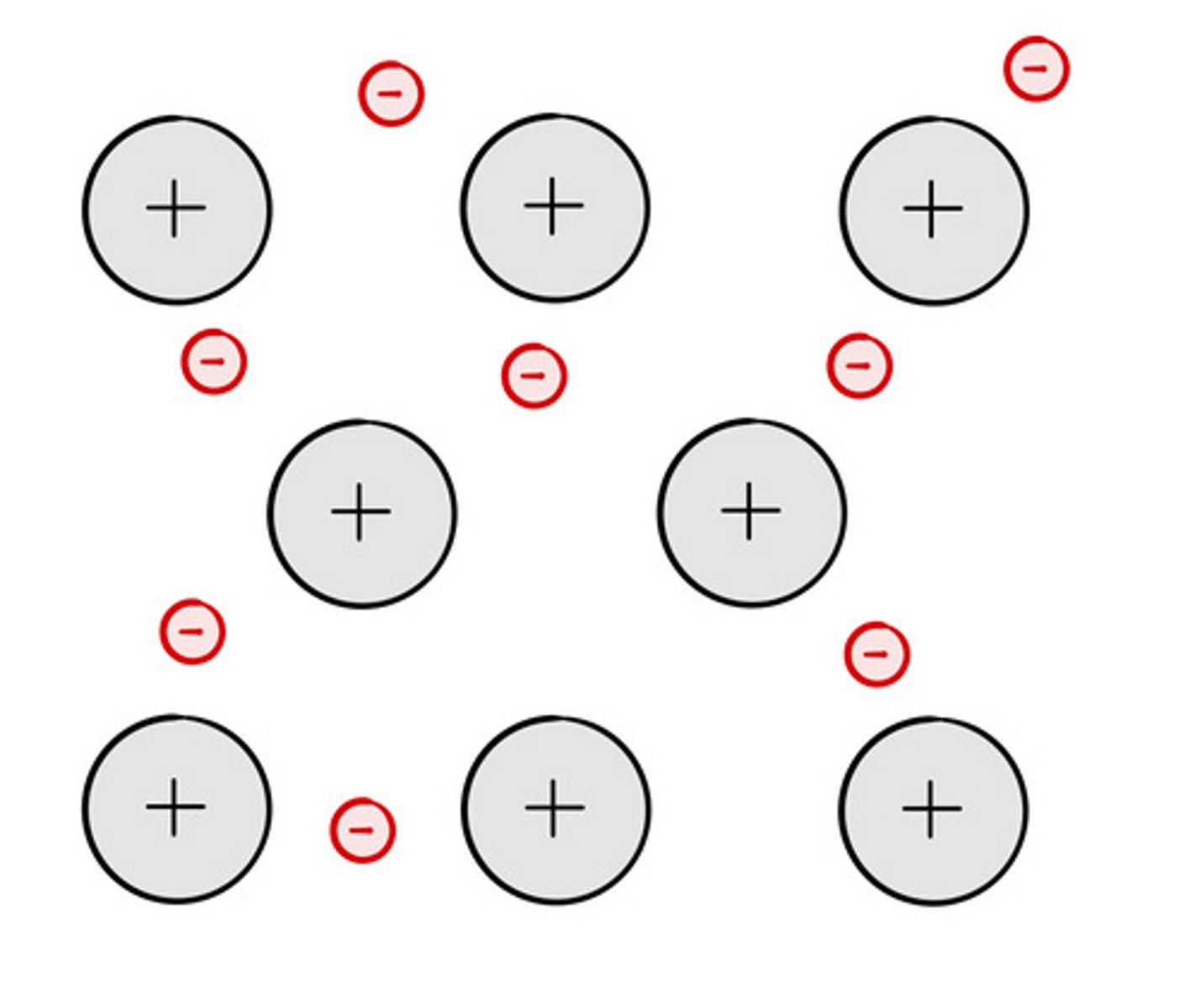
Electrostatic forces of attraction
Strong forces of attraction between oppositely charged particles
Elements that form ionic compounds
Metals and non-metals together
Elements that form covalent bonds
non-metals
Elements that form metallic bonds
metals and alloys

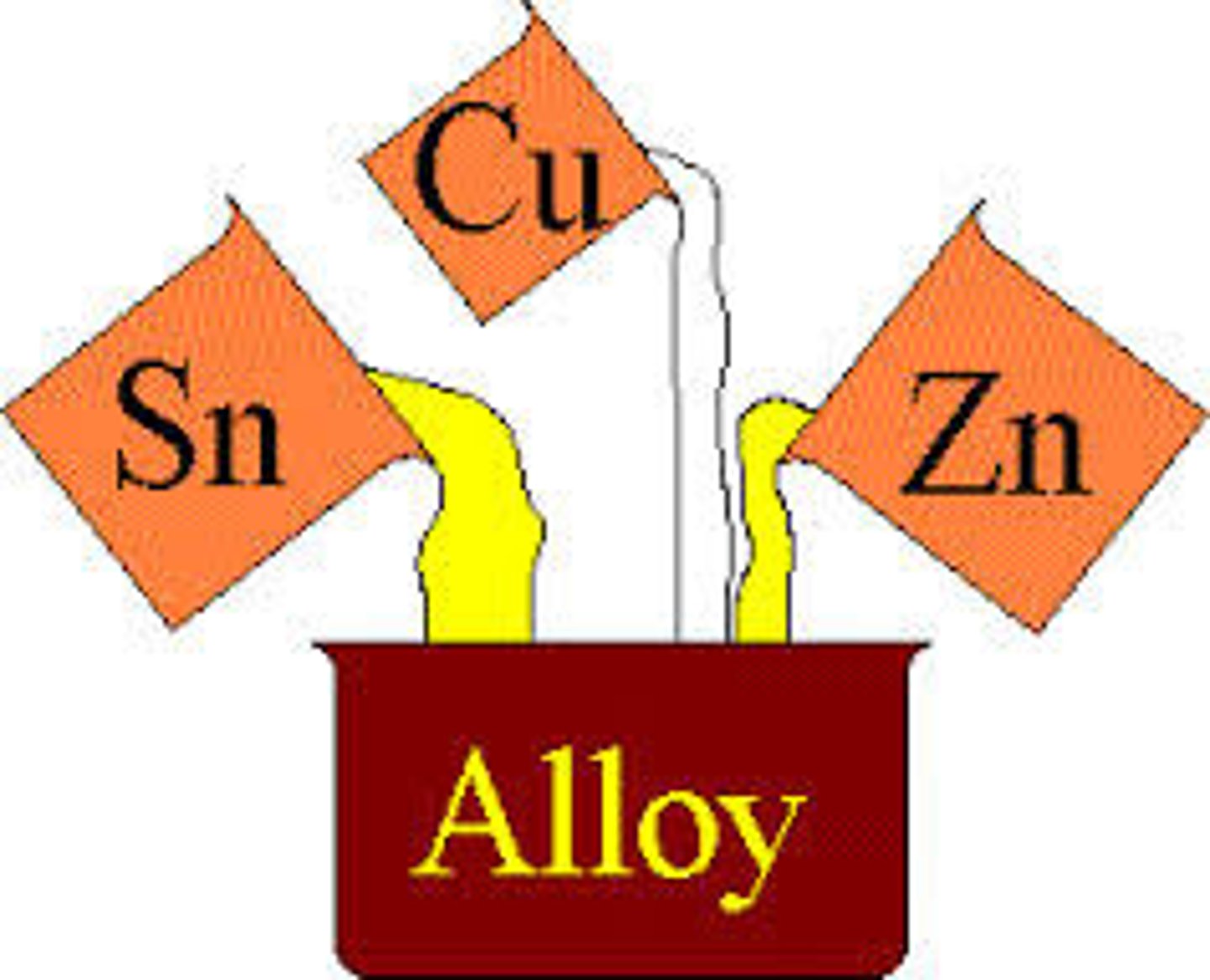
Alloy
A metal mixed with other elements