Molecular Geometry and VSEPR — Flashcards from Lecture Notes (Video)
1/14
Earn XP
Description and Tags
A set of Q&A flashcards covering common molecular geometries (linear, bent, trigonal planar, tetrahedral, trigonal pyramidal, seesaw, square planar, square pyramidal, octahedral) and their associated bond counts, lone pairs, and typical angles as stated in the notes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
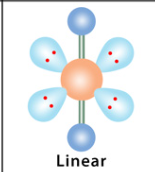
What is the geometry and bond angle for a molecule with 2 bonds and 0 lone pairs?
Linear geometry with a 180° bond angle.

What is the geometry and bond angle for a molecule with 3 bonds and 0 lone pairs?
Trigonal planar geometrywith a bond angle of 120 degrees.
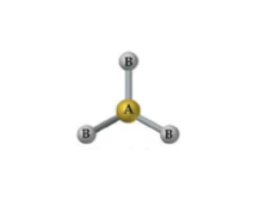
What is the geometry and approximate bond angle for a molecule with 2 bonds and 1 lone pair?
Bent geometry with an angle less than 120°.

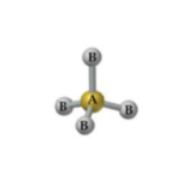
What is the geometry and bond angle for a molecule with 4 bonds and 0 lone pairs?
Tetrahedral geometry with a 109.5° bond angle.
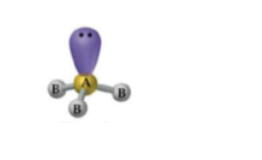
What is the geometry and approximate bond angle for a molecule with 3 bonds and 1 lone pair?
Trigonal pyramidal geometry with an angle less than 109.5°.
According to the notes, what is the geometry and angle for 2 bonds and 2 lone pairs?
Trigonal bipyramidal geometry with an angle less than 109.5°.
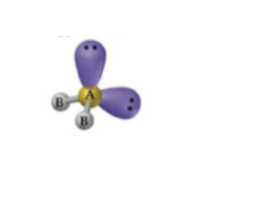
For 5 bonds and 0 lone pairs, what geometry and angles are listed?
Seesaw geometry with 90° and 120° angles.
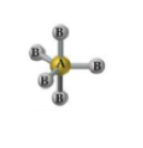
What is the geometry and angle for 4 bonds and 1 lone pair?
T-shaped geometry with angles less than 90° and less than 120°.
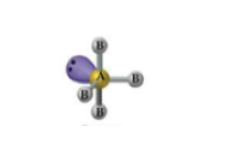
What is the geometry and angle for 3 bonds and 2 lone pairs?
Trigonal bipyramidal
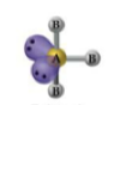
What is the geometry and angle for 2 bonds and 3 lone pairs?
Octahedral geometry with a 180° angle.

What is the geometry and angle for 6 bonds and 0 lone pairs?
Octahedral geometry with 90° angles.
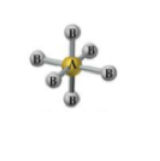
What is the geometry and angle for 5 bonds and 1 lone pair?
Square planar geometry with an angle less than 90°.

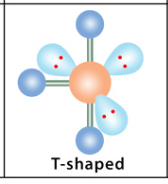
What is the geometry and angle for 4 bonds and 2 lone pairs?
T-shaped geometry with 90° angles.

For 3 bonds and 3 lone pairs, what is the geometry and angle?
T-shaped geometry
What is the angle for a molecule with 2 bonds and 4 lone pairs?
linear 180°.