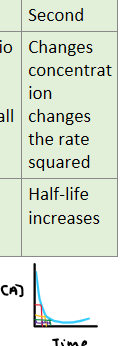Rate equations and orders + half life
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What is the rate equation? Also what can be said about the rate constant?
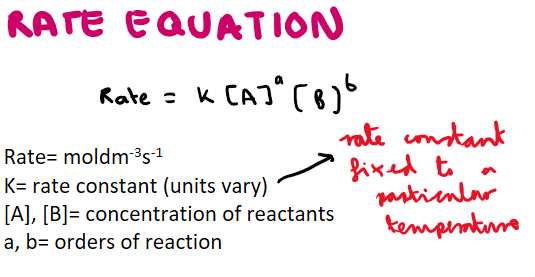
What happens to rate constant as temperature increases?
Rate constant increases as temperature does as the reactant particles will have more kinetic energy and collide more often (increasing the rate)

What are the different types of orders of reaction?
Zero
First
Second
Define zero order
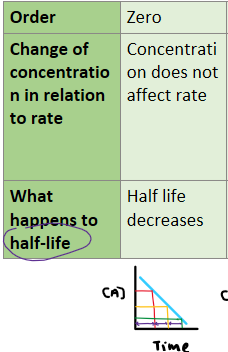
Change in concentration of reactant will have no effect on rate. Rate is constant.
Define first order
Changes in concentration has a proportional change on rate.
E.g. [A] x 2 -> rate x 21
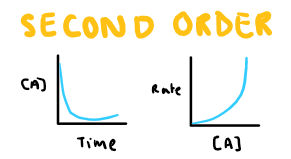
Define second order
Changes in concentration has a squared proportional change on rate.
E.g. [A] x2 -> rate x 22
How do you work out orders of reactants?
Repeat the experiment several times but change concentrations of reactants one at a time.
Then work out initial rate of the reaction for each experiment via a graph.
Draw up a table with information on concentration of all reactants and their initial rates in each experiment.
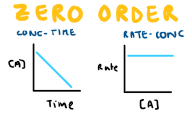
Draw the conc-time and rate-conc graph for zero order
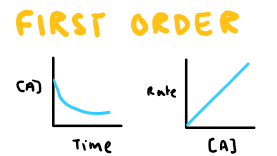
Draw the conc-time and rate-conc graph for first order
Draw the conc-time and rate-conc graph for second order
What is the half-life of a reaction?
Half-life: the time taken for half the reactant to be used up

How can you identify the half-life of reactions?
By using conc-time graphs
What happens to the half life of a reaction as time progresses for a zero order reaction?
What happens to the half life of a reaction as time progresses for a first order reaction?
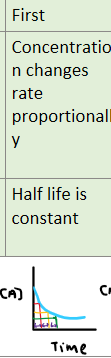
What happens to the half life of a reaction as time progresses for a second order reaction?