Chapter 12, Cellular Energies: Chemiosmosis, Electron Transport, Glycolysis, Mitochondria
1/92
Earn XP
Description and Tags
Bolded words for slides defined by textbook glossary
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
93 Terms
Citric Acid and Glyoxylate cycle
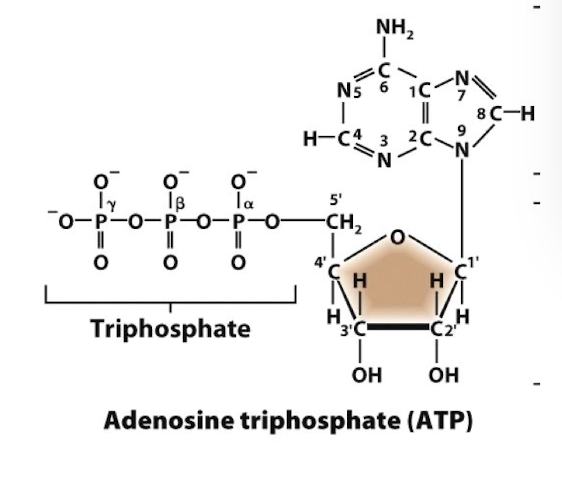
Structure of ATP
A ribose sugar: not deoxyribose, not the -OH on C2’
An adenine base (purine) bound to C1’
3 phosphoryl groups esterified to one another via phosphoanhydride bonds on C5’- this is where the energy iss
The electronegative charges of the phosphoryl groups repel one abother making the bonds inherently unstable and at a “high energy” level.
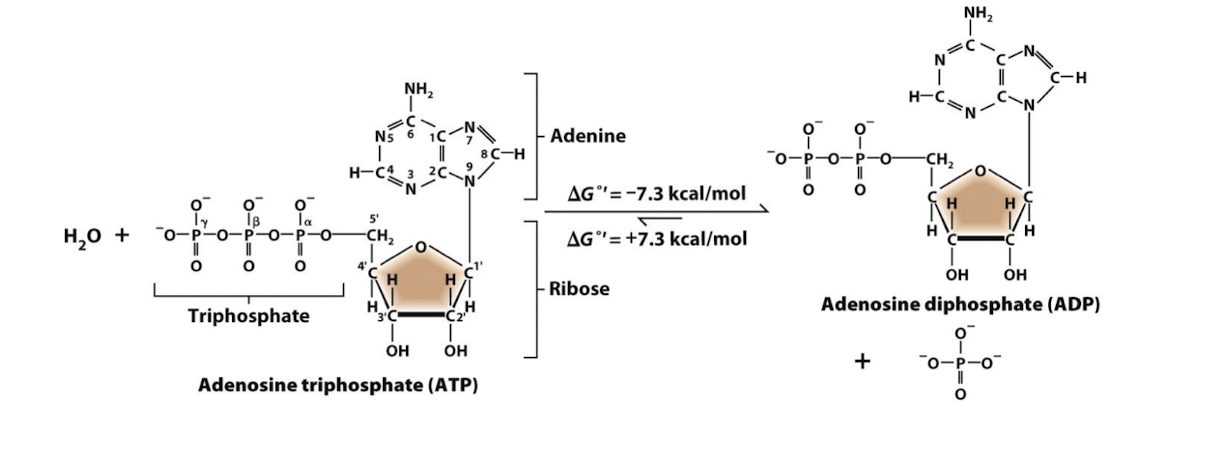
ATP hydrolysis
The formation of more stable bonds in the products ADP and orthophosphate
usually involves an enzyme
∆G˚’= -7.3 kcal/mol
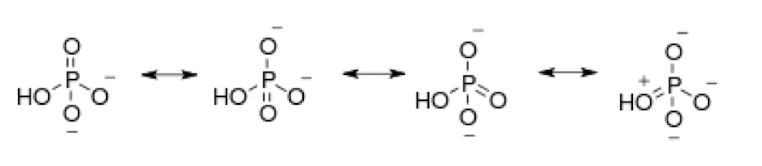
Orthophosphate
The multiple resonance structures of orthophosphate make it a very stable low energy molecule.
Aerobic respiration
Reduced organic molecules are oxidized to generate high energy reduced electron carriers (NADH and FADH2) that power oxidative phosphorylation- the synthesis of ATP from ADP and Pi.
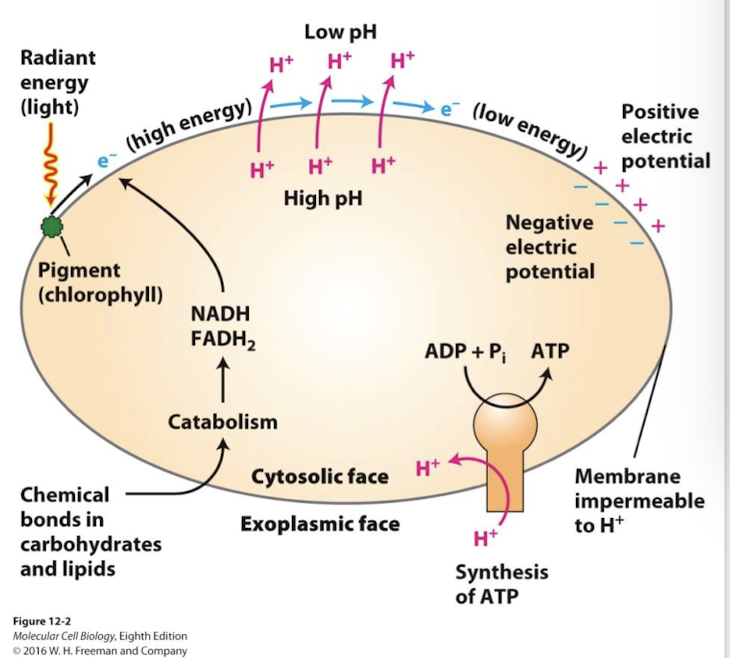
Oxidative Phosphorylation
An electron transport chain pumps H+ across a membrane to create H+ electrochemical gradient: A proton-moving force. The energy of the proton-motive force drives ATP synthesis by powering the F-type ATP pump called ATP synthase.
Chemiosmosis
The diffusion of ions (usually H+ ions) across a selectively permeable membrane through transporters.
Chemiosmosmotic coupling is the use of energy released from this movement to drive other cellular processes.
Aerobic oxidation
Cells use a four-stage process to transfer energy released by glucose/fatty acid oxidation into the terminal phosphoanhydride bonds of ATP.
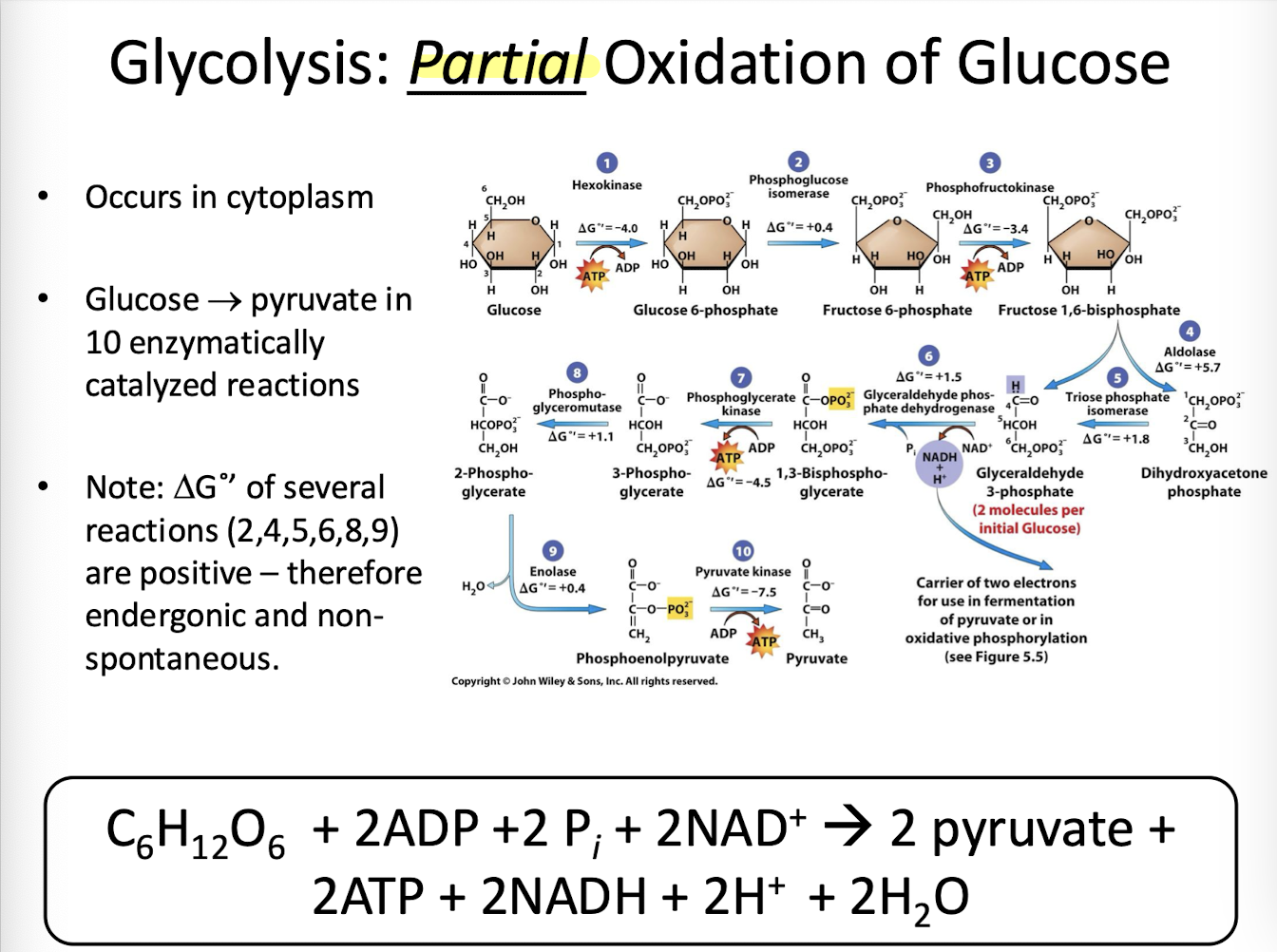
Glycolysis
Stage 1 of aerobic oxidation. Cytosolic enzymes partially oxidize glucose to two molecules of pyruvate and generate two molecules each of NADH and ATP. Glycolysis does not require oxygen and is therefore an anaerobic process. Occurs in the cytoplasm of eukaryotic cells.
Most of the energy produced is released as heat
The remaining energy is ultimately captured in the newly formed terminal phosphoanhydride bonds of ATP.
Fermentation
In the absence of oxygen some cells can reduce pyruvate to lactic acid or ethanol/CO2 to oxidize NADH back to the NAD + needed to drive glycolysis.
Redox reactions
(oxidation-reduction reactions) involve a transfer of electrons between two species (atoms or molecules)
Two half reactions
an oxidation reaction
a reduction reaction
Species being oxidized donates electrons to the species being reduced- aka electron acceptor
when a substrate loses electrons, it is oxidized (LEO)
when a substrate gains electrons, it is reduced (GER)
Redox in Glycolysis and Citric Acid Cycle
During glycolysis and the citric acid cycle carbon atoms form sugars, fats, and amino acids are oxidized- they give up their grip on shared electrons through successive enzymatically catalyzed Redox reactions.
Metabolism
The collection of all biochemical reactions that occur within a cell.
Metabolic pathways
Sequential chemical reactions linked by enzyme catalyzed steps in order to produce or degrade specific molecule.
Catabolic Pathways
Break down complex substates down into simple products. (Spontaneous, exergonic)
provides raw materials for cellular processes
transfers chemical energy from complex molecules to the high energy bonds of ATP and other high-energy activated carriers
Anabolic pathways
synthesize complex end products from simple substrates (Non-spontaneous, endergonic)
require the energy of ATP and other high-energy activated carriers
Most also require reducing agents
Citric Acid Cycle
Completes the oxidation of glucose and other metabolites. It is the second stage of cellular respiration and occurs in the mitochondrial matrix of eukaryotic cells (aka the Tricarboxylic Acid Cycle or Kreb’s Cycle).
Reducing Agents
Activated energy carriers
These are molecules that give up electrons to some other chemical species in a Redox reaction.
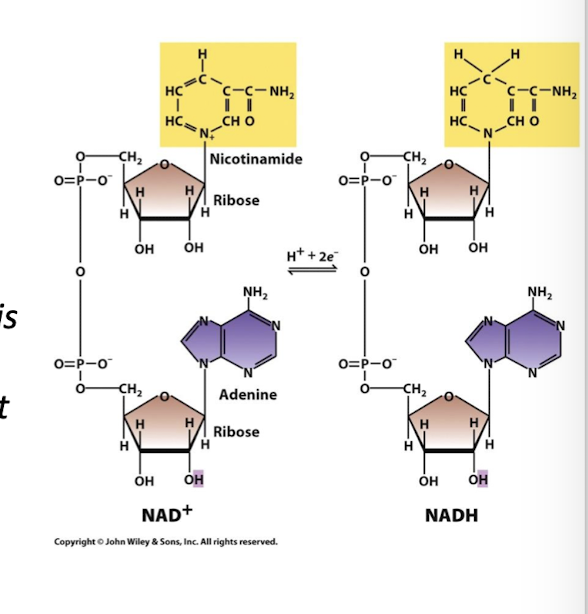
Most central to ATP synthesis: Nicotinamide Adenine Dinucluotide (NAD+) (oxidized) and NADH (reduced)
NADH’s function in cellular metabolism
Only one function, it donates its electrons to the first electron acceptor of the Electron Transport Chain
When NAD+ is reduced it accepts two e- and one H+ from the enzyme substrate.
FAD+ is reduced to FADH2 by accepting two e- and two H+ from the enzyme substrate.
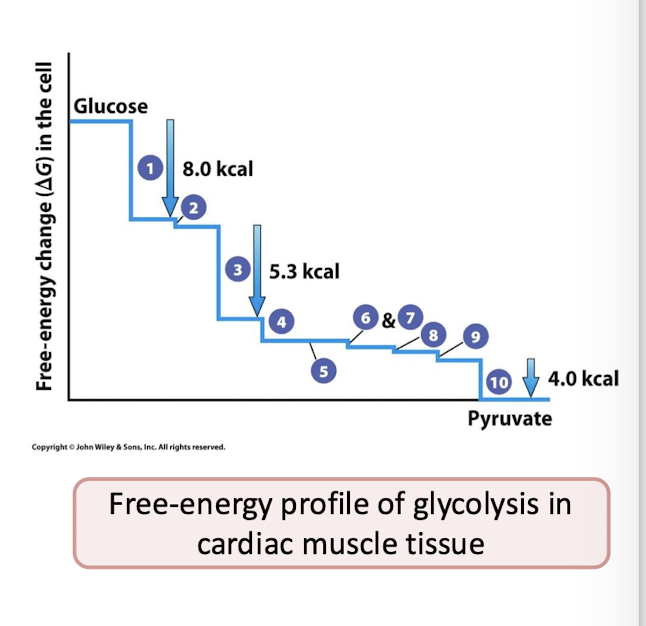
Why in cells is the actual ∆G of glycolysis favorable?
Products are being consumed by the next step of the pathway, keeping the forward direction going.
The irreversible reactions: Three of these reactions have very large -∆G values. This makes them essentially irreversible under normal conditions and they drive the entire process in the forward direction.
Irreversible reactions are 1,3, and 10
Glycolysis Step 1
Energy Investment Phase
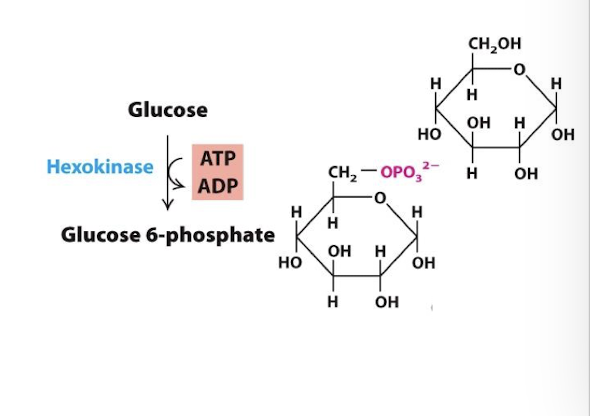
The first irreversible step: Glucose is phosphorylated to generate glucose 6-phosphate
This raises the energy level of glucose metabolites in subsequent reactions
By changing glucose to G-6-P the cytoplasmic concentration of glucose stays low- GLUT transporters can continue transporting glucose into cell
Enzyme: Hexokinase
Allosteric Regulation: The product, glucose—6-phosphate, is an allosteric inhibitor of hexokinase. Negative feedback regulation: an excess of G-6-P pauses pathway at its first step
Glycolysis Step 2
Energy Investment Phase
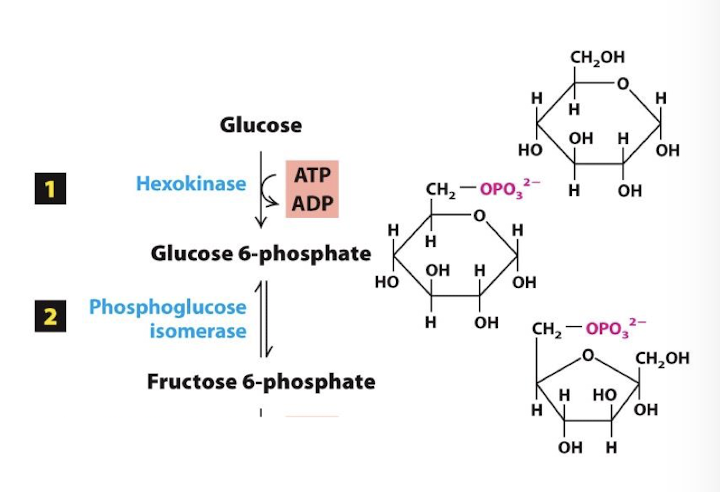
Glucose 6-phosphate is isomerized to fructose 6-phosphate. This conversion of a six-sided sugar ring to a five-sided ring sets the stage for producing a nearly symmetrical molecule in the next step.
Pentose Phosphate Pathway
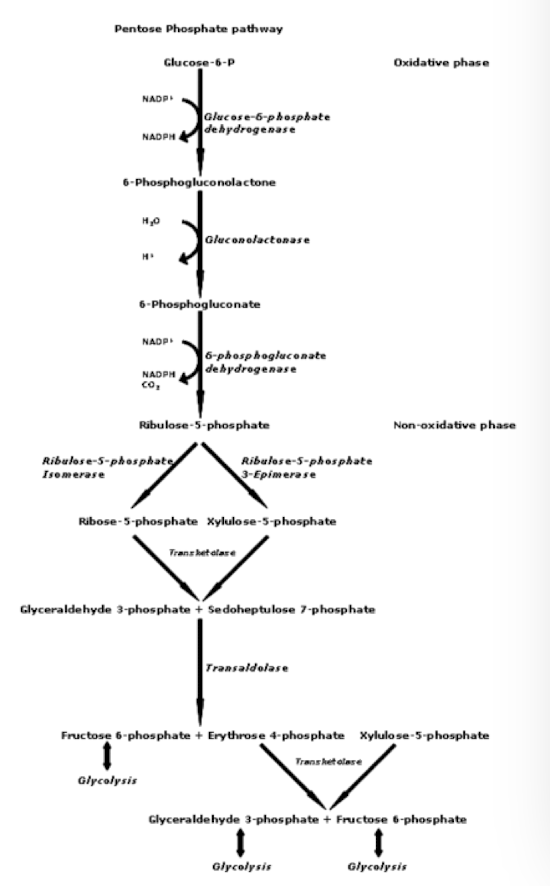
Is a metabolic pathway that runs parallel to glycolysis
It uses glucose-6-phosphate as a starting material and generates:
NADPH: the principal reducing agent in the cytoplasm for anabolic processes like synthesis of fatty, amino, and nucleic acids.
Ribose 5-phosphate a precursor for the synthesis of nucleotides
Glycolysis Step 3
Energy Investment Phase
In the second irreversible step Fructose 6-phosphate is phosphorylated to generate fructose 1,6-bisphosphate (F1,6bP). Another ATP is consumed in this step.
Committed step: a term for an irreversible step in an enzymatic pathway for which the product will be used solely for completion of that pathway.
Rate limiting step of glycolysis.
Enzyme: Phosphofructokinase-1- most important control enzyme in the glycolytic pathway and its acctivity is regulated by several positive and negative influences.
The doubly phosphorylated F1,6bP is nearly symmetrical, so that its hydrolysis in the next step yields two very similar molecules.
Allosteric Regulation:
allosterically activated by AMP positive feed-back regulation. High AMP indicates there is low ATP, so this accelerates glycolysis.
allosterically activated by Fructose 2,6 bisphosphate (F2, 6bP) which production is regulated by insulin and glucagon- hormones released by pancreatic beta and alpha cells, respectively, in response to high blood sugar (insulin) or low blood sugar (glucagon) → insuling promotes F2,6bP which promotes glycolysis and Glucagon inhibits F2,6bP which inhibits glycolysis
allosterically inhibited by ATP and citrate (the first metabolite of the CAC) negative feed-back regulation. When there is lots of product available for cellular respiration, why process F-6-P? instead, it and G-6-P can instead be fed into other pathways, like Pentose Phosphate Pathway.
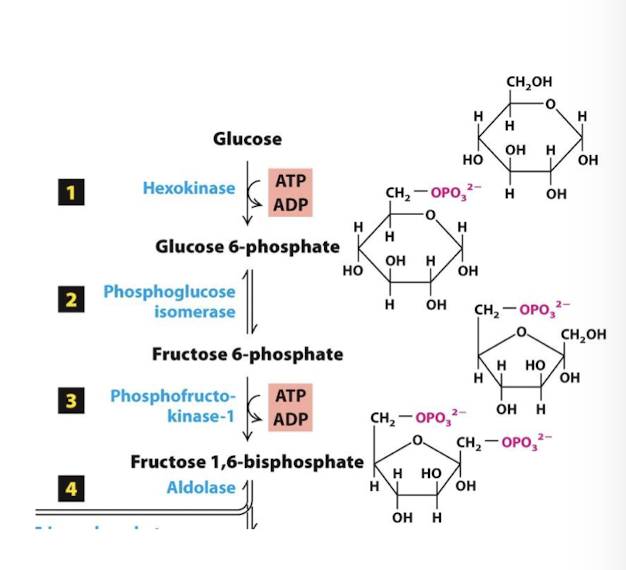
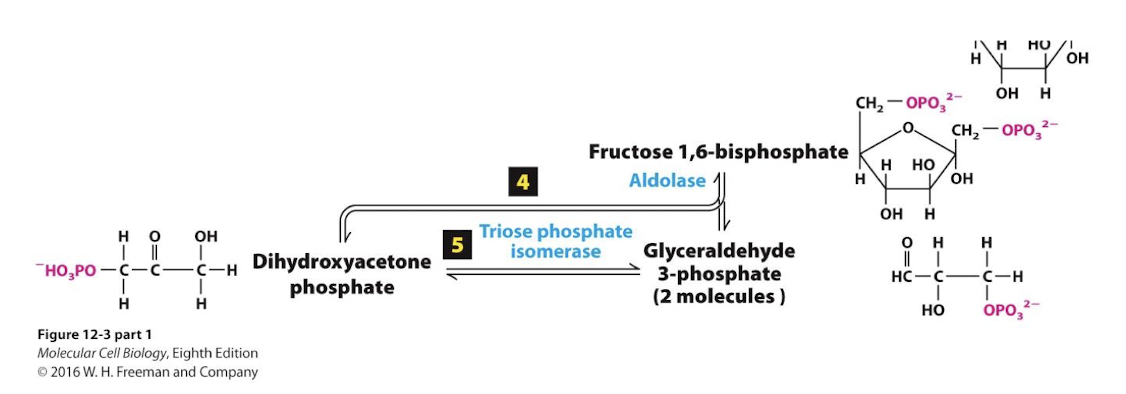
Glycolysis Step 4
Cleavage Phase
F1,6bP is split into 3 carbon molecules glyceraldehyde 3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP)
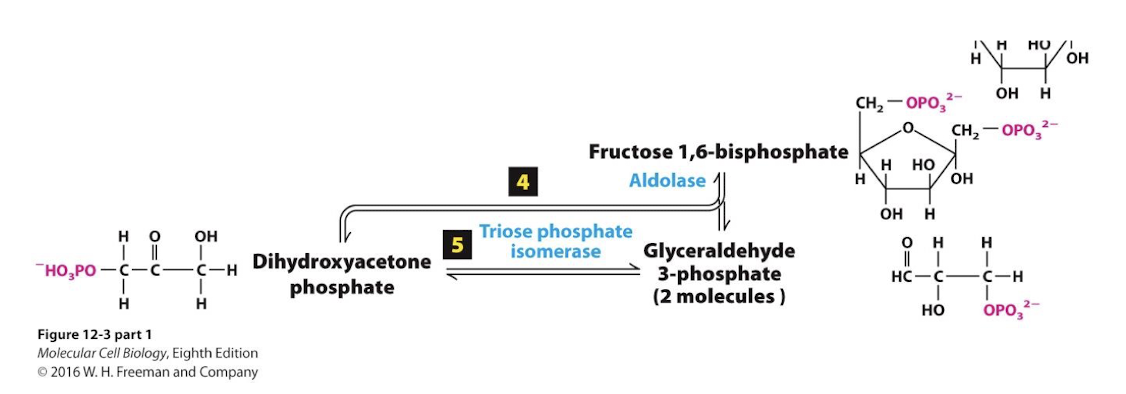
Glycolysis Step 5
Cleavage Phase
An isomerase interconverts the two molecules and G3P is siphoned off by step 6.
note there are now 3-carbon molecules representing the remaining oxidation potential of the original 6-carbon glucose.
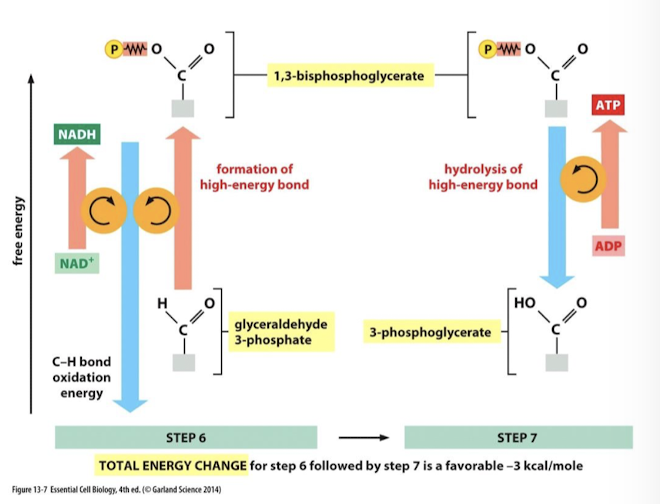
Glycolysis Step 6
The Energy Payoff Phase
Oxidation of G3P is strongly exergonic. It is coupled to the reduction of NAD+ to NADH and the phosphorylation of G3P to make 1,3-bisphosphoglycerate.
Enzyme: glyceraldehyde 3 phosphate dehydrogenase (dehydrogenase: oxidize substate and reduce cofactors or coenzymes. NAD+ is the coenzyme for this dehydrogenase) catalyzes three sequential reactions it couples the release of energy from carbon oxidation to drive the two subsequent reactions.
G3P is oxidized→ favorable
A proton and 2 e- from its cabonyl carbon are transferred to, and reduce, NAD+ to NADH
G3P is phosphorylated on its oxidized carbon with an inorganic phosphate from cytoplasm: 1,3 bisphosphoglyverate is the product.
Because the 6 carbon glucose has been split into 2 3 carbon molecules, this reaction must take place twice to process one glucose equivelent. Therefore, 2 NADH are generated per glucose molecule.
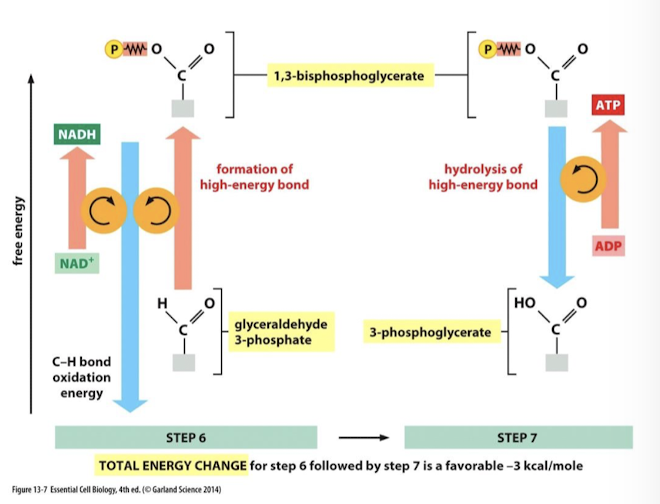
Glycolysis Step 7
The Energy Payoff Phase
Hydrolysis of a phosphate bond in 1,3-bisphosphoglycerate is energetically favorable (has sufficient transfer potential to donate its phosphate group) and is coupled to phosphorylation of ADP generating ATP.
Enzyme: phosphoglycerate kinase
Substrate Level Phosphorylation: the production of ATP or GTP by the transfer of a phosphate group from a substrate directly to ADP or GDP
Small amout of ATP is generated by substrate level phosphorylation:
4 molecules total per molecule of glucose
2 at step 7, and 2 at step 10
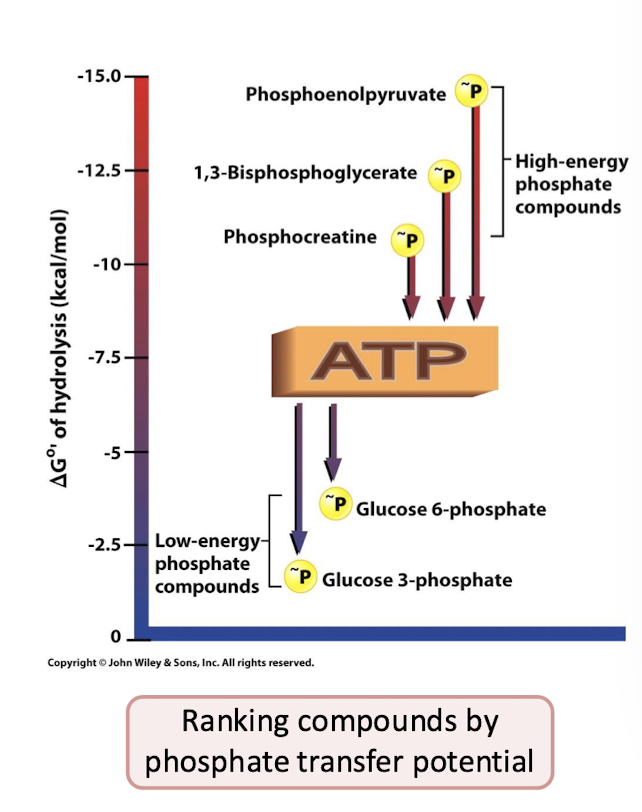
Transfer potenital
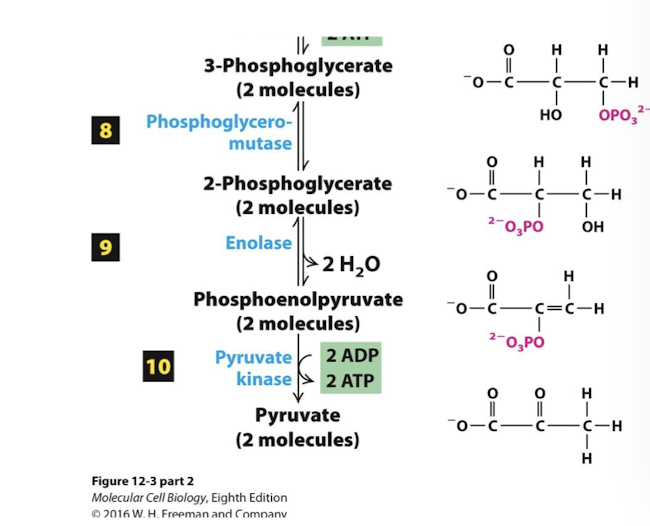
Glycolysis Steps 8-9
The Energy Payoff Phase
Generate transfer potential: 3-phophoglycerate is converted to phosphoenolpyruvate, a molecule with enough transfer potential to make ATP.
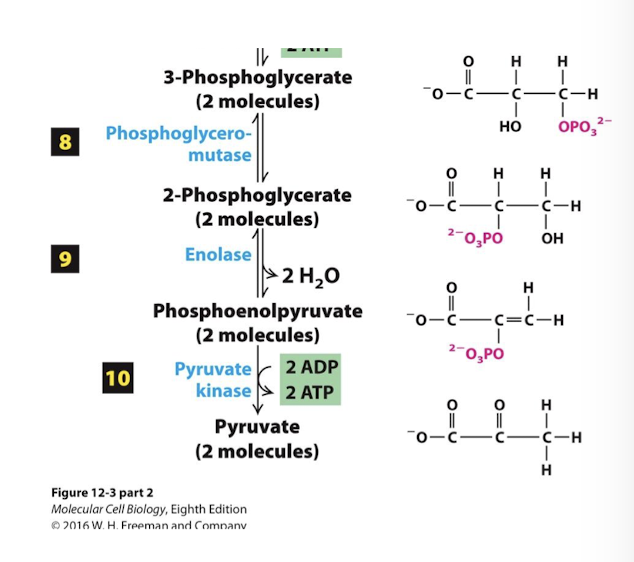
Glycolysis Step 10
The Energy Payoff Phase
In the fina irreversible step pyruvate kinase catalyzes the transfer of the phosphate group from phosphoenolpyruvate to ADP generating 2 additional ATP per glucose via substrate level phosphorylation.
Final product of glycolysis, pyruvate, is generated.
Allosteric regulation:
Pyruvate kinase is allosterically inhibited by ATP
It is allosterically activated by F1, 6bP (the product of step 3)
Structure and of oxidation state of pyruvate
One carbon remains highly oxidized
The other two are highly reduced
Net Equation for Glycolysis
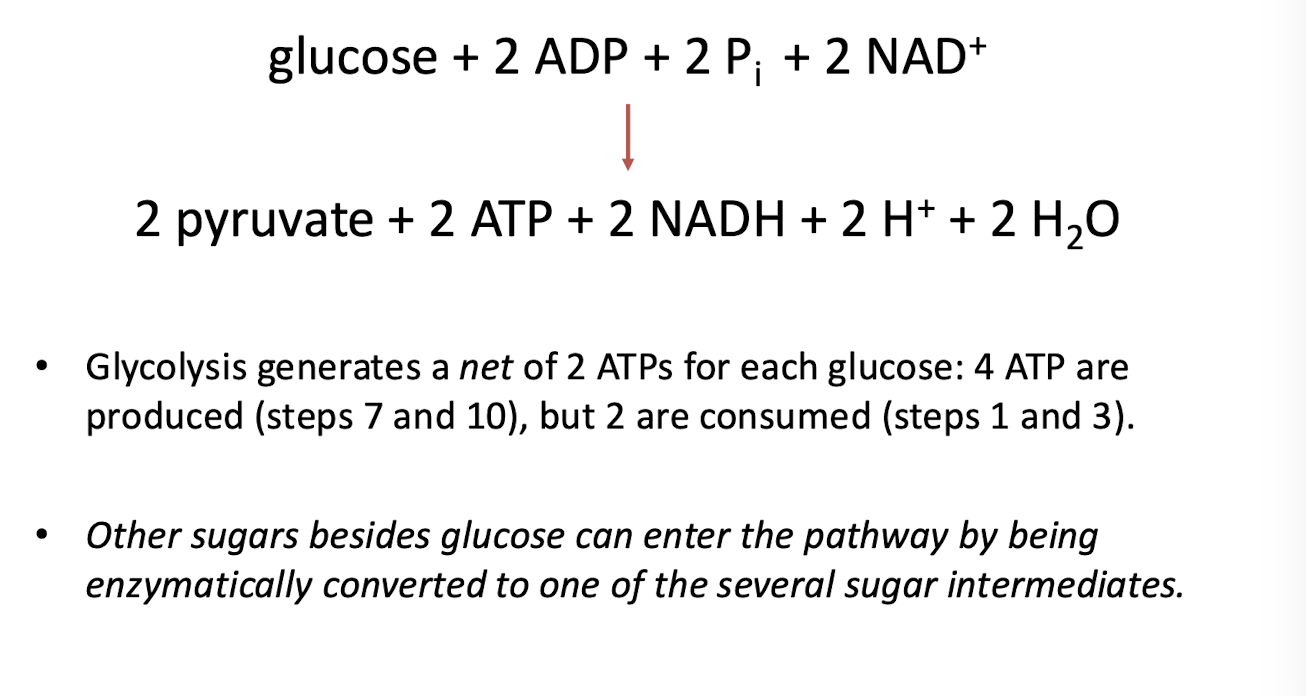
What happens after glycolysis
Glycolysis occurs in the absence of oxygen, it is an anaerobic pathway.
The end product, pyruvate, can enter either aerobic or anaerobic catabolic pathways.
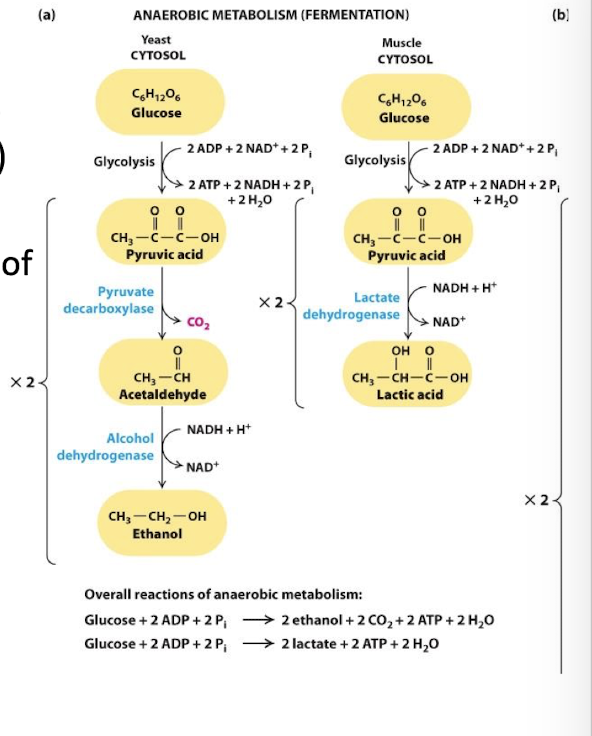
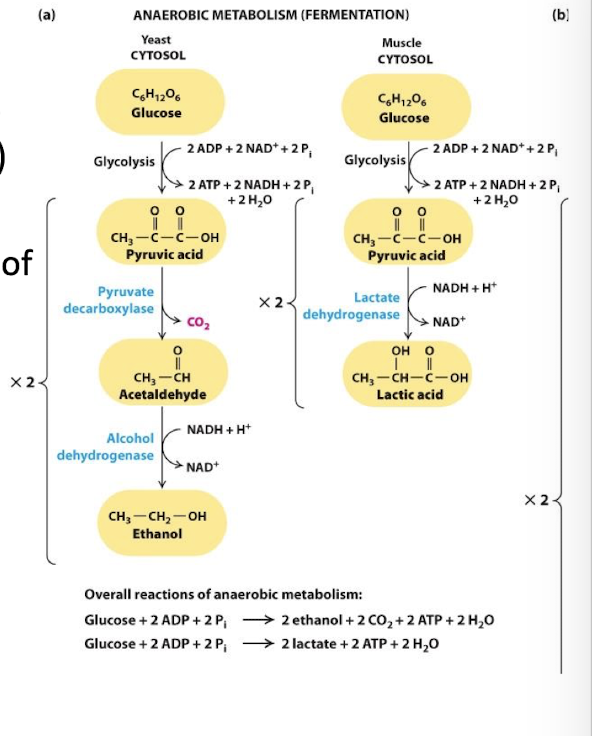
Anaerobic Oxidation of Pyruvate: The Fermentation Process
Fermentation restores cytosolic NAD+ from NADH:
under anaerobic conditions, glycolysis is the only source of ATP
Glycolysis depletes cytoplasmic supplies of NAD+ by reducing NADH
Without NAD+ to drive step 6 no glycolysis
fermentation NADH is oxidized to NAD+ coupled to the reduction of pyruvate
Lactic Acid Fermentation:
Fermentation coupled to reduction of pyruvic acid directly to lactic acid
Enzyme: Lactate dehydrogenase
Bacteria and animal embryonic, muscle, and tumor cells
Alcohol Fermentation:
pyruvate is first decarboylated to acetaldehyde releasing Co2
Acetaldehyde is reduced to ethanol coupled to the oxidation of NADH
Enzyme: Alcohol dehydrogenase
Yeast and other microbes
Mitochondrial Structure and Function: Outer membrane
Porin VDAC (voltage dependent anion channel) most abundant protein. Permeable to ions and small proteins allowing free exchange between intermembrane space and cytoplasm
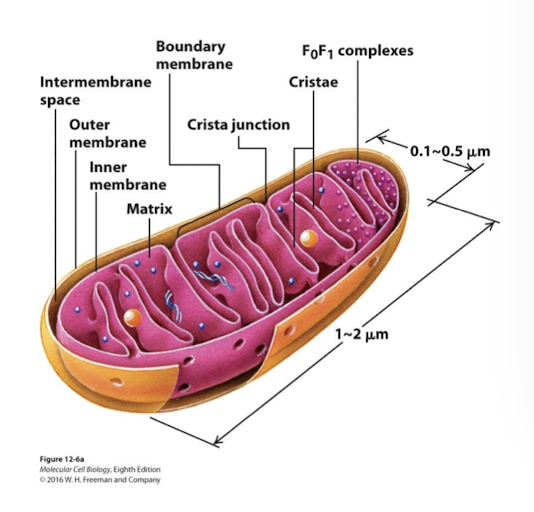
Mitochondrial Structure and Function: Inner membrane
Inner mitochondrial membrane is the major permeability barrier to the mitochondrial matrix. It has two interconnected domains:
Boundary membrane: Adjacent to outer membrane- held in contact by protein complexes called MICOs
Cristae: Interior folds where the protein complexes involved in ATP synthesis are located
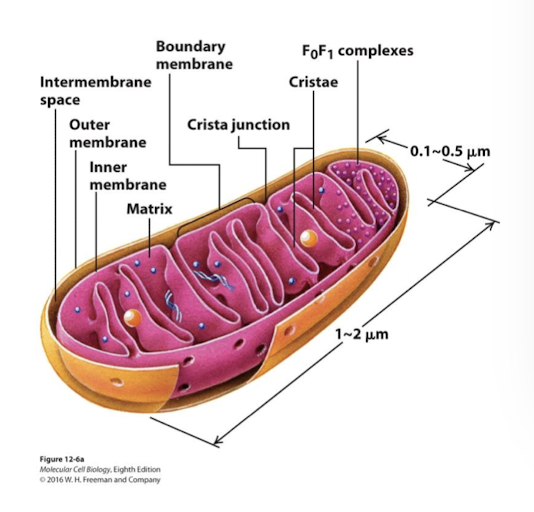
Mitochondiral Structure and Function: Intermembrane space
Between the two membranes into which protons are pumped to generte the proton-motive force
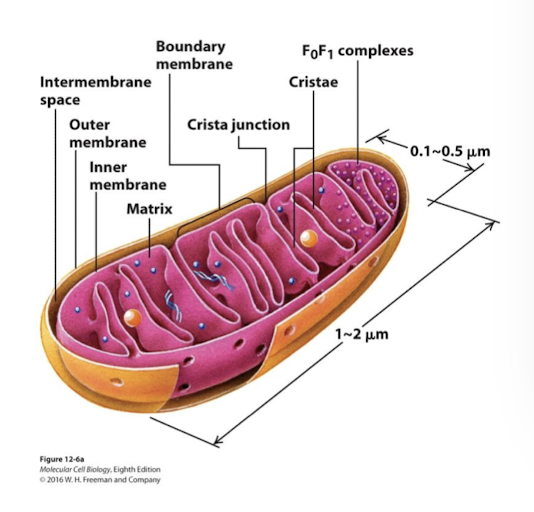
Mitochondrial Structure and Function: Matrix
Interior of the inner membrane. Contains multiple copies of the circular mt chromosome, ribosomes, and diverse enzymes.
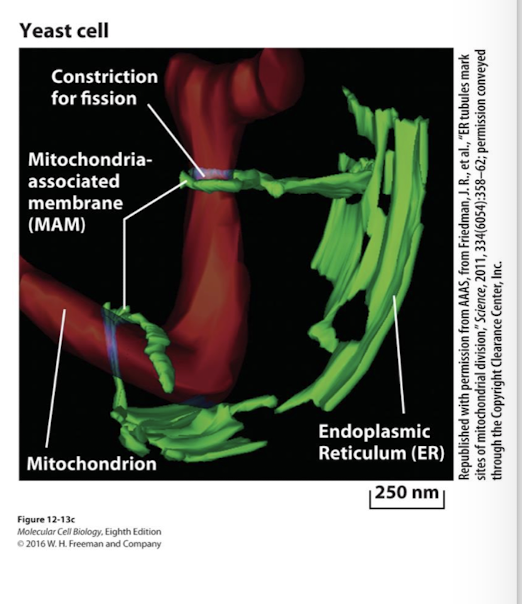
ER mitochondria associated membranes (MAMs)
Regulate mt fusion and fission
calcium influx from MAMs to mitochondria stimulate ATP synthesis because some Citric Acid Cycle enzymes are Ca2+ dependent
helps activate signals from mitochondria that promote apoptosis
Important Concepts: The Citric Acid Cycle and Fatty Acid Oxidation
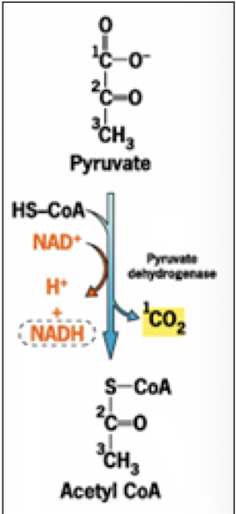
The mitochondrial matrix complex pyruvate dehydrogenase links glycolysis with the Citric Acid Cycle by oxidizing pyruvate, reducing NAD+ and releasing acetyl-CoA into the matrix.
Acetyl-CoA is further oxidized to 2 molecules of CO2 in the Citric Acid Cycle- Releasing additional electron carriers NADH and FADH2
Most of the energy in oxidation stages I and II is temporarily stored in reduced NADH and FADH2 which carry high-energy electrons that subsequently drive Electron Transport Chain.
Oxidation of short- to long-chain fatty acids occurs in mitochondria with production of ATP and additional NADH
Cytoplasmic NADH donates electrons to shuttle pathways that ferry them to mitochondrial matrix
Anaerobes
organisms that capture and utilize energy by oxygen-independent metabolism.
Evolved to us O2 in order to extract more energy from organic molecules- it is the most electronegative biological element
Cyanobacteria
Oxygen accumulated into the primitive atmosphere after cyanobacteria evolved and began to photosynthesize, increasing O2 levels in the atmosphere
Eukaryotic aerobic reparation takes place where?
the mitochondria
Stage I of Aerobic Oxidation of Glucose and Fatty Acids
Stage I: Glycolysis: partial glucose oxidation, FA-CoA synthesis
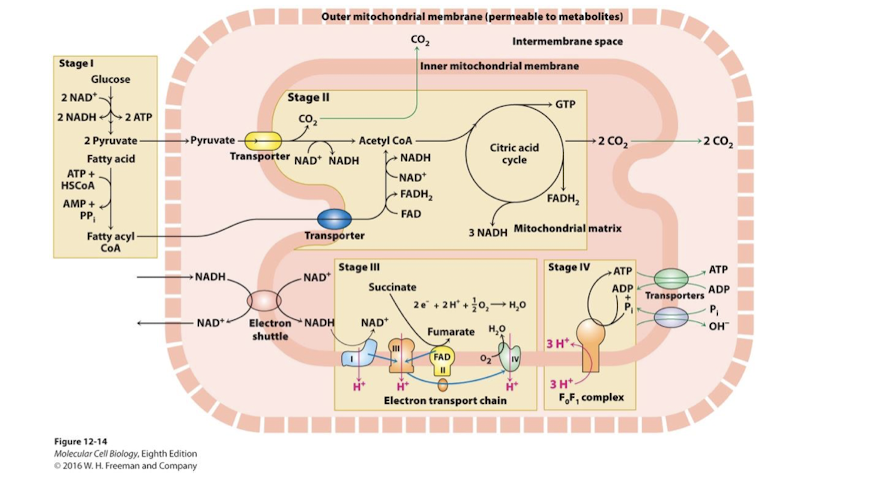
Stage II of Aerobic Oxidation of Glucose and Fatty Acids
Stage II: Mitochondrial matrix: Pyruvate Dehydrogenase, Citric Acid Cycle and Fatty Acid oxidation
pyruvate is oxidized to acetyl CoA while NAD+ is reduced to NADH
acetyl CoA is oxidized to CO2 more NAD+ is reduced to NADH
fatty acyl CoA is oxidized releasing acetyl CoA and additional FADH2/NADH
a small amount of ATP or GTP is synthesized via substrate level phosphorylation
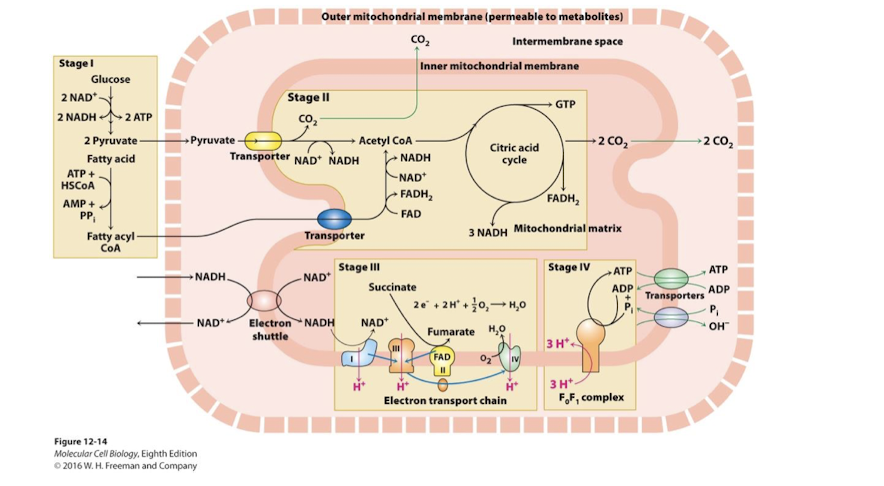
Stage III of Aerobic oxidation of Glucose and Fatty Acids
Stage III: Inner Membrane: Electron Transport and Proton Motive Force
NADH and FADH2 are oxidized
their electrons pass through ETC to the ultimate oxidizing agent O2
energy released by sequential ReDox reactions drives H+ pumping from matrix to inter-membrane space- this generates the proton-motive force
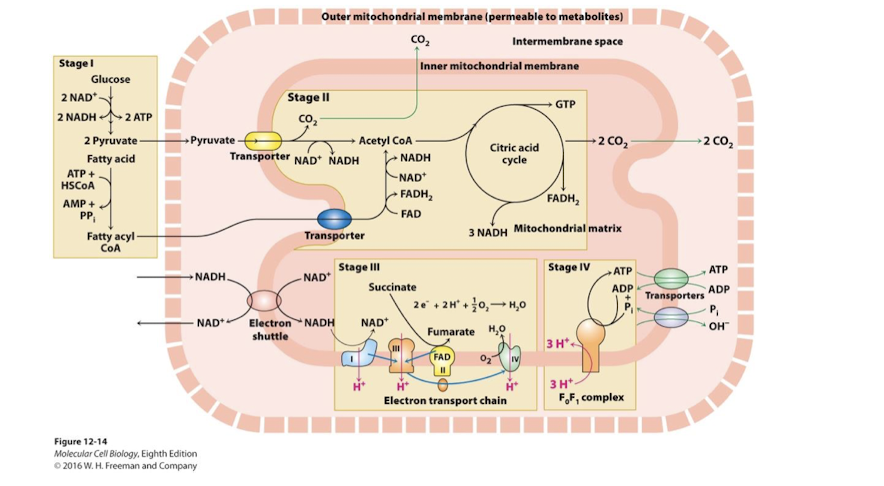
Stage IV of Aerobic oxidation of Glucose and Fatty Acids
Stage IV: Inner Membrane and Matrix: Oxidative Phosphorylation
ATP synthase harnesses proton-motive force to generate ATP in the matrix
Antiporter proteins- import ADP and Pi into the matrix coupled to export of ATP and hydroxide ions
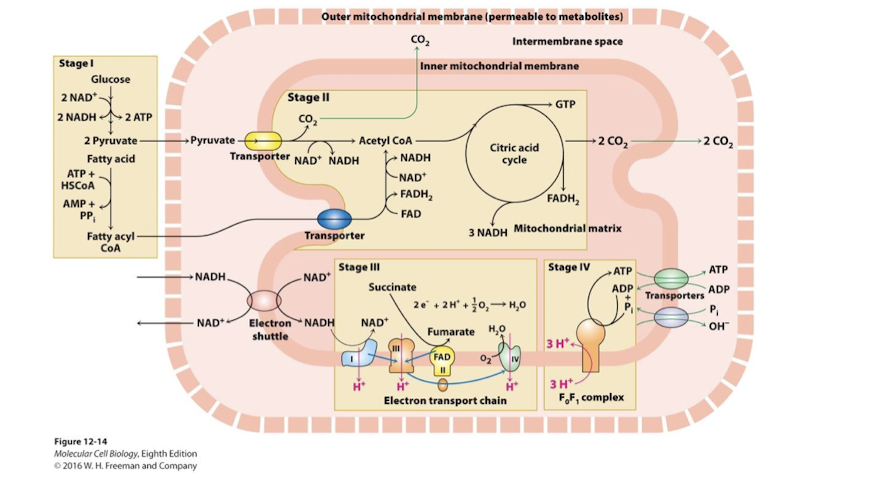
Oxidative Metabolism in the Mitochondrion: Pyruvate Dehydrogenase Complex links Glycolysis to TCA cycle
Pyruvate Dehydrogenase Complex links Glycolysis to TCA cycle
Mitochondrial inner membrane symporter pyruvate translocase uses the proton-motive force to couple H+ diffusion into the matrix with the import of pyruvate
Three Catalytic Steps in the Pyruvate Dehydrogenase Complex:
Oxidizes pyruvate, releasing CO2- the first completely oxidized carbon from a glucose molecule
Oxidation is exergonic and coupled to: Acylation of Coenzyme A forming acetyl CoA
Oxidation is exergonic and coupled to: Reduction NAD+ to NaDH
Catalyzed by glyceraldehyde-3-phosphhate dehygrogenase
Pyruvate Dehydrogenase complex: A complex of three enzymes that converts pyruvate into acetyl-CoA. Acetyl CoA links glycolysis to TCA cycle through the action of the Pyruvate Dehydrogenase Complex
Allosteric Regulation
Positive: Fructose-1,6 bisphosphate as well as ER derived Ca2+
Negative: NADH and acetyl CoA
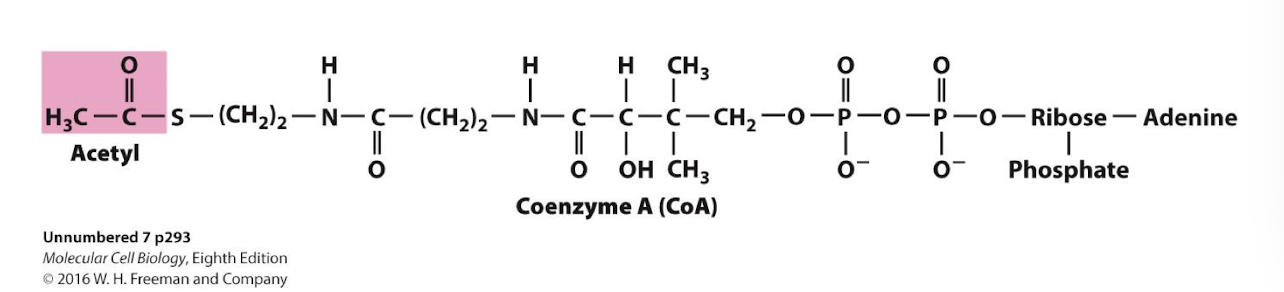
Acetyl-Coenzyme A
An activated energy carrier: the thioester bond is very unstable and will readily break and form the more stable -SH bond, while transferring the acetyl group to another molecule, likewise form a most stable new bond.
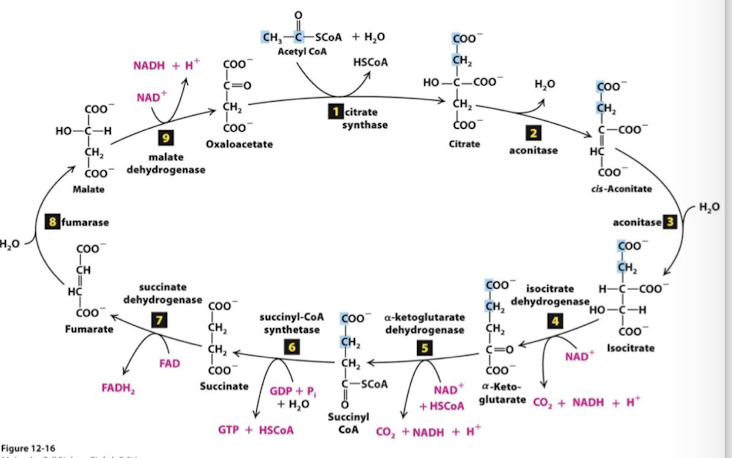
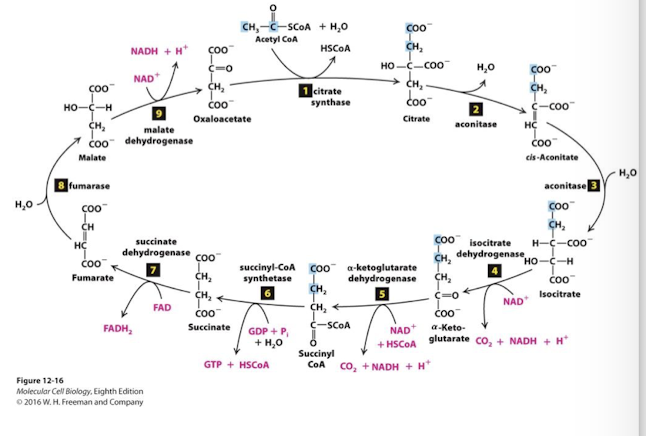
Oxidative Metabolism in the Mitochondrion: Citric Acid Cycle (Krebs Cycle)
9 sequential enzymatic steps complete the oxidation of one acetyl equivalent
4 additional pairs of electrons are removed from substrate molecules by dehydrogenase enzymes of the TCA cycle
oxidation steps 4,5,7,and 9 are accompanied by the reduction of 3 NAD+ and FAD
the final metabolite is oxaloacetate, the 4-carbon molecule that initiates the cycle
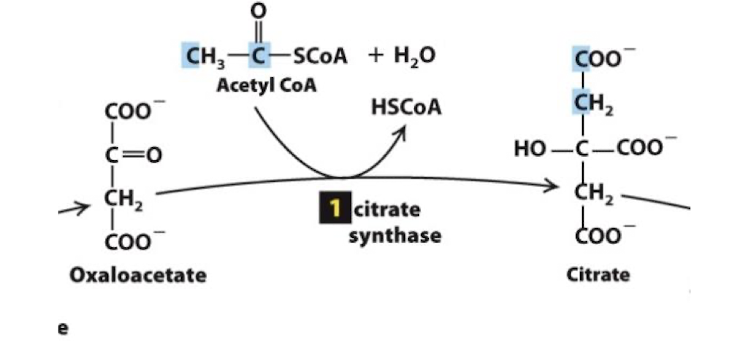
Citric Acid Cycle Step 1
The enzyme citrate cynthase catalyzes condensation of the two-carbon acetyl group with the four-carbon oxaloaxetate to form a six-carbon citrate molecule. Extremely exergonic and irreversible step.
Transfer potential of the Acetyl CoA thioester bond drives this reaction. CoA is reduced and released as HSCoA.
Citric Acid Cycle Steps 2 &3
2 and 3. Isomerization of citrate into iscocitrate: The tertiary alcohol citrate is not readily oxidized, but the secondary alcohol isocitrate is .
similar to steps 2, 8, and 9 of glycolysis, convalent bonds are rearranged to make the metabolite more reactive- in this case making a stronger reducing agent- isocitrate
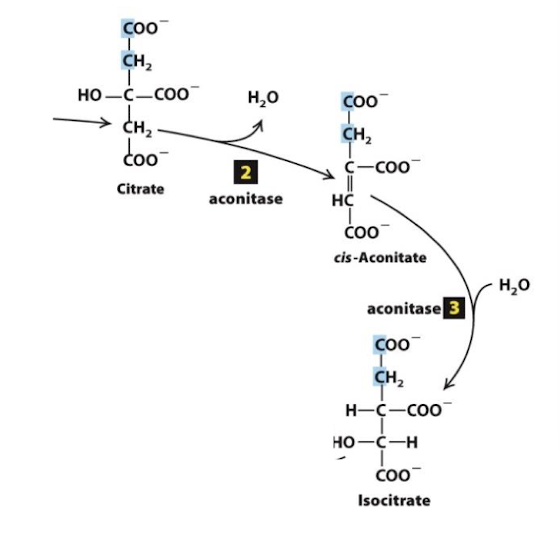
Citric Acid Cycle Step 4
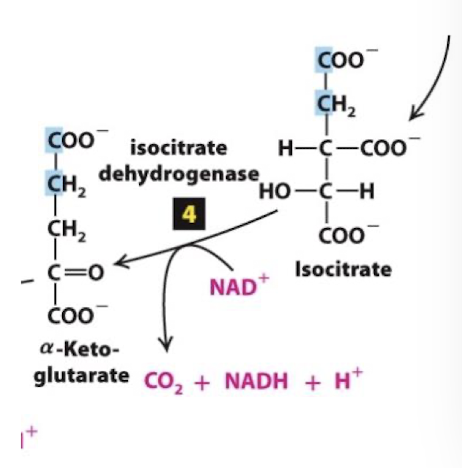
Reduction of the first electron carrier:
Isocitrate is oxidized to the 5-carbon alpha-ketogutarate
one molecule of CO2 fully oxidized carbon is released
NAD+ is reduced to NADH
catalyzed in a 3-step process by the enzyme isocitrate dehydrogenase
irreversible step with a large -∆G
rate liminting step of CAC
Allosteric Regulation:
Negative: ATP, NADH, alpha-ketoglutarate
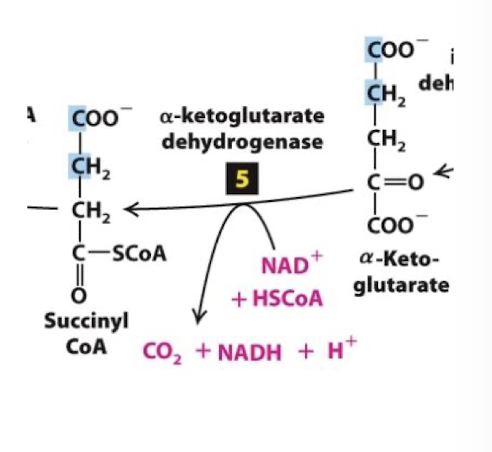
Citric Acid Cycle Step 5
The alpha-ketoglutarate dehydrogenase complex catalyzes oxidation of alpha-ketoglutarate to succinyl CoA.
A third carbon is completely oxidized- so one full pyruvate equivalent has been oxidized at this point
Alpha-ketoglutarate dehydrogenase is analogous to pyruvate dehydrogenase: its oxidation energy drives:
Reduction of NAD+ to NADH
Thioester bond formation between CoA and succinate forming succinyl CoA
Highly exergonic, tightly regulated, irreversible reaction.
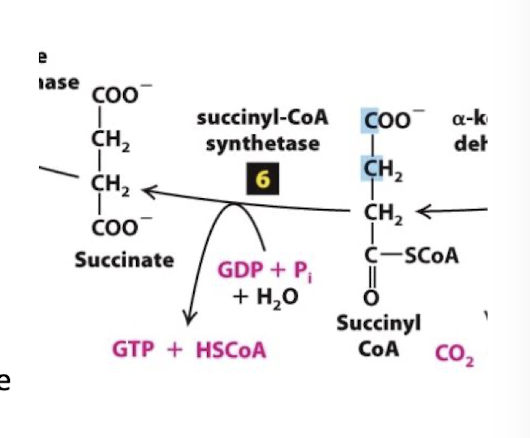
Citric Acid Cycle Step 6
Substrate-Level Phosphorylation: The transfer potential of Succinyl-CoA formation of an energy-rich phosphate intermediate
The enzyme succinyl-CoA synthetase catalyzes replacement of CoA by an inorganic phosphate:
Hs-CoA is released
Succinyl is phosphorylated
Transfer potential of succinyl phosphate is sufficient to drive phosphorylation of a nucleotide diphosphate
In plants and bacteria, ATP is formed directly in this step. In animals, GTP is generated, but is counted as an ATP equivalent.
Citric Acid Cycle Step 7
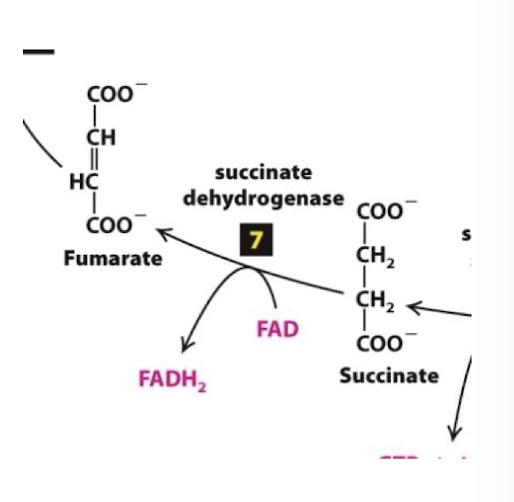
Flavin-dependent dehydrogentation: Succinate is oxidized to fumarate, reducing FAD to FADH2. Flavin adenine dinucleotide
FAD< a coenzyme and electron carrier similar to NAD+, accepts two H+ and 2 electrons and is reduced to FADH2.
the enzyme succinate dehydrogenase is a part of one of the four complexes of the Electron Transport Chain
the complex succinate dehydrogenase participates in both TCA and ETC
Citric Acid Cycle Step 8
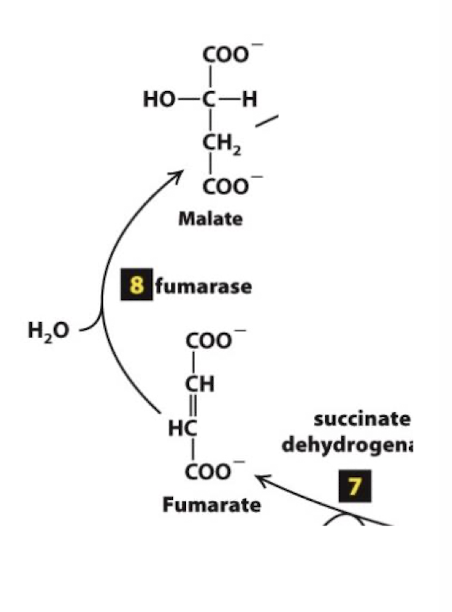
Hydration of a C=C bond: Fumarate is hydrolyzed to form malate.
Similar to steps 8 and 9 of Glycolysis, this rearrangement makes the metabolite more reactive. In this case the rearrangement generates a better reducing agent, malate.
Citric Acid Cycle Step 9
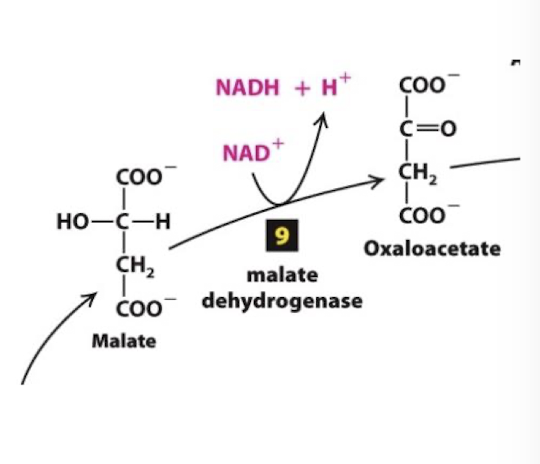
The cycle is complete when the final enzyme malate dehydrogenase catalyzes oxidation of malate to oxaloacetate and the corresponding reduction of another NAD+ to NADH
Highly endergonic reaction > +7 kcal/mol that moves in the forward direction because the extremely exergonic citrate synthesis reaction keeps oxaloacetate concentrations very low.
4 ReDox Reactions in TCAC for each per acetyl-CoA
2C released as CO2- completing oxidation of one pyruvate equivalent
so two turns of this cycle completes the oxidation of one glucose equivalent
3 reactions: NAD+ reduced to NADH
1 ATP equivalent generated
1 reaction: FAD reduced to FADH2
The function of TCAC is not to yield large quantities of ATP
The cycle produces high energy carrier molecules (NADH and FADH2) that power the synthesis of ATP
Oxidative Metabolism in the Mitochondrion: The Malate Aspartate Shuttle
NADH genergated in the cytosol has no utility. Cytosolic enzymes that require reducing power use NADPH (produced by the PPP)
Glycolysis requires a constant supply of NAD+ in the sytoplasm to drive oxidation of glyceraldehyde-3-phosphate. Without NAD+, glycolysis will come to a screeching halt.
Cytosolic NADH must be oxidized to NAD+ so glycolysis can use this critical co-enzyme to drive the payoff stages
Two shuttle systems function to oxidize cytosolic NADH and carry its H+ and electrons to the mutochondrial matrix.
Malate Aspartate Shuttle
Oxidizes cytoplasmic NADH and reduces matrix NAD+- major shuttle in liver, heart, and kidney
Importane:
The electrons captured by glycolytic redox reactions can be used to drive the ETC
A cytoplasmic supply of NAD+ is maintained
Key: By keeping malate concentrations high in the matrix and oxaloacetate high in the cytosol, the dehydrogenases and transaminases drive the same reactions in reverse directions
Glycerol Phosphate Shuttle
Oxidizes cytoplasmic NADH and reduces matrix FAD to FADH2- most body cells
Importane:
The electrons captured by glycolytic redox reactions can be used to drive the ETC
A cytoplasmic supply of NAD+ is maintained
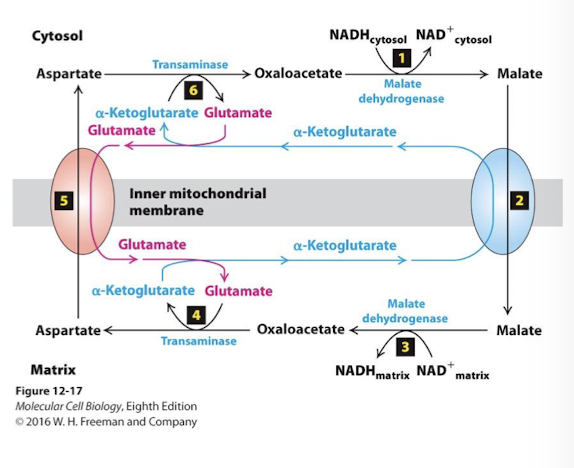
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 1
The key enzyme is malate dehydrogenase (which catalyzes step 9 of TCA). In the cytosol it catalyzes the reverse reaction- oxidation of NADH and reduction of oxaloacetate to malate.
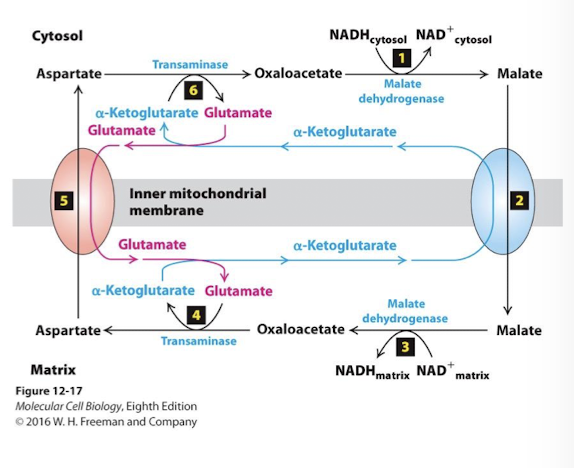
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 2
An antiporter in the inner membrane pumps malate into the mitochondrial matrix using the power of the alpha-ketoglutarate concentration gradient
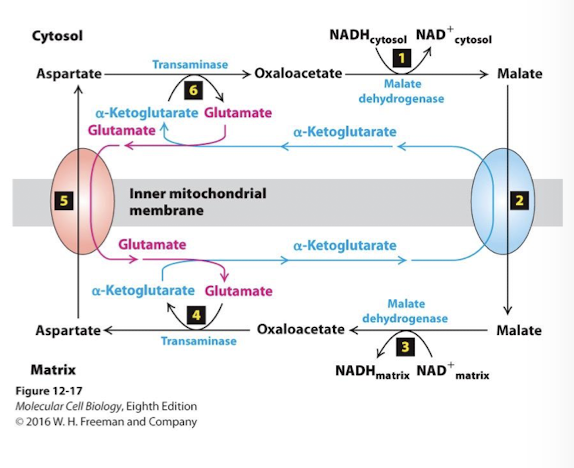
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 3
In the matrix, malate dehydrogenase reduces NAD+ to NADH while oxidizing malate to oxaloacetate.
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 4
IN the matrix, the enzyme transaminase transfers the amino group of glutameate to oxaloacatate yielding aspartate and alpha-ketoglutarate
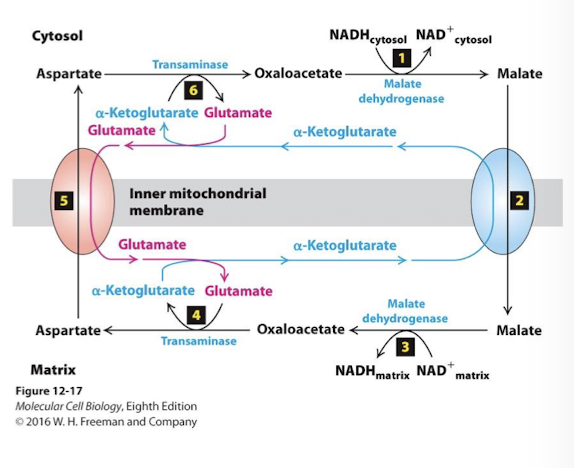
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 5
A second antiporter pumps aspartate to the cytoplasm using the power of the glutamate gradient.
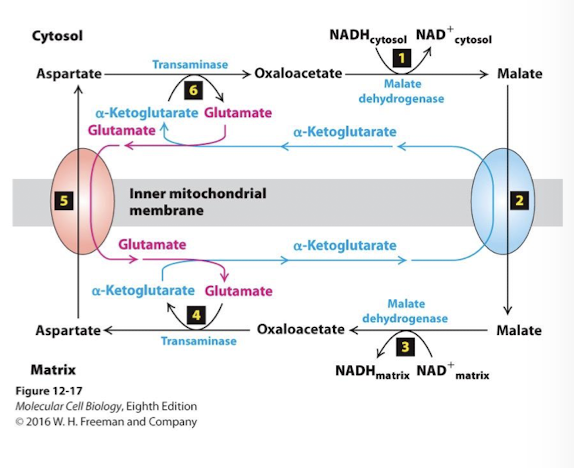
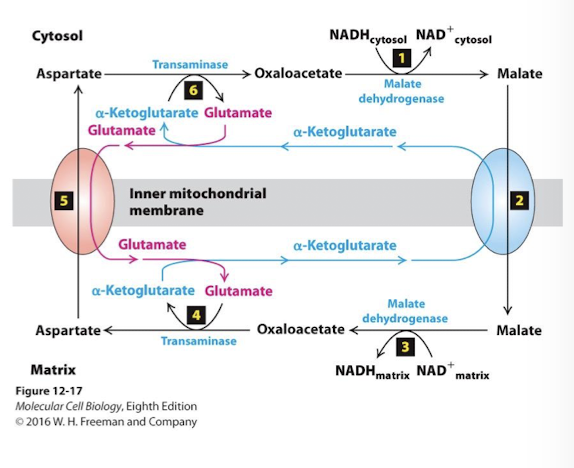
Malate Aspartate Shuttle NADH/NAD+ ReDox Step 6
Glutamate concentration is kept high in the cytosol though the action of the same transamine- rinning in reverse - it converts aspartate to oxaloacetate by transferring the amide to alpha-ketoglutarate yielding glutamate
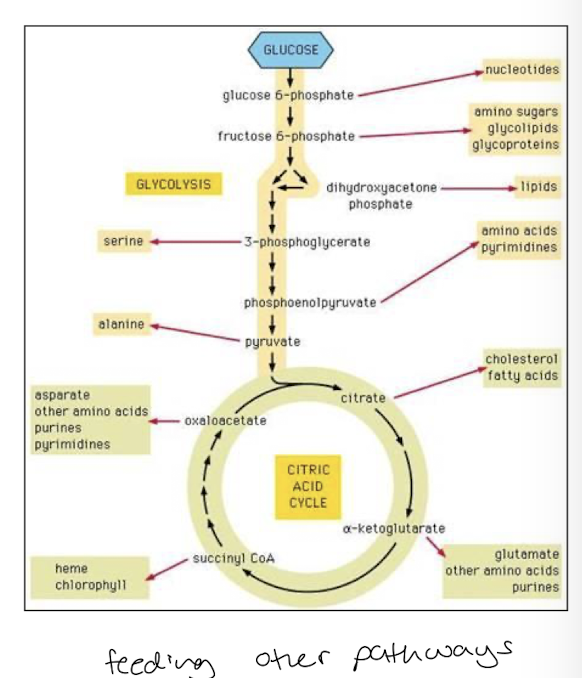
TCAC central metabolic pathway of the cell
Reaction intermediates of TCAC are common metabolites of other catabolic reactions
many pathways feed metabolites into TCAC at various steps
Metabolic intermediates of glycolysis and TCAC are substrates for diverse anabolic pathways synthesizing:
Amino Acids
Nucleotides
Lipids
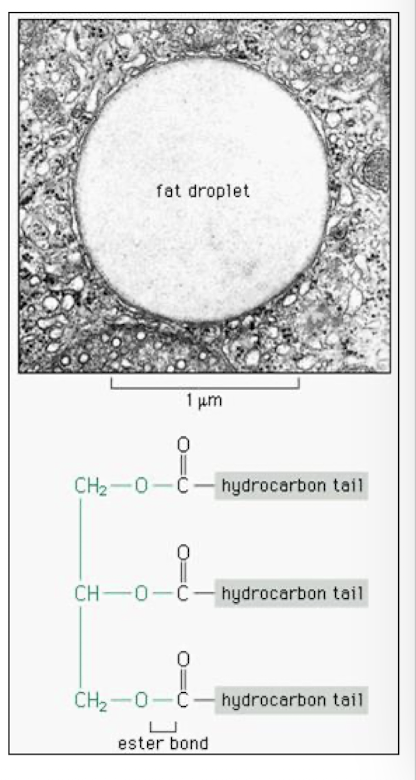
Fats: Triacylglyceridea
Significant source of energy- hydrocarbons contain highly reduced carbons
stored as triglycerides in lipid droplets in adipose cells. upon glucagon stimulation fatty acids are cleaved from glycerol and released into the blood stream
transported into cells and in ATP dependednt manner esterified to CoA
Oxidative Metabolism in the Mitochondrion: Beta-oxidation: The Fatty Acid Cycle
2 dehydrogenase enzymes sequentially oxidize carbon 3 of the acyl-CoA. Each turn of the cycle reduces one FAD and one NAD+.
another enzyme cleaves acetyl CoA and joins another CoA to the shortened chain
released acetyl CoA is fed to TCAC
Each turn of the Fatty Acid Cycle produces:
4 NADH (1 from FA cycle and 3 from CAC)
2 FADH2 (1 from FA cycle and 1 from CAC)
1 ATP equivalent (from CAC)
Peroxisomes
Membrane-bounded vesicles that contain > 50 oxidative enzymes that catabolize diverse biomolecules
Use o2 to oxidize these substrates, producing hydrogen peroxide H2O2 as a bi-product
peroxisomal enzyme catalyase converts this toxic molecule into O2 and H2O
Site of plasmalogen synthesis, a lipid enriched in cardiovascular and myelin cell-functioning to insulate excitable cells.
As a function in cellular respiration:
Beta-oxidation of very long chain fatty acids (VLCFAs): FA tails longer than 22 carbons
The shorter FA-CoA molecules are then transported to mitochondria where Beta-oxidation is completed.
Oxidative Phosphorylation
A 2 Step Process:
Electron Transport Chain estblishes a H+ electrochemical gradient: proton-motive force
Flow of protons drives ATP production via ATP synthase
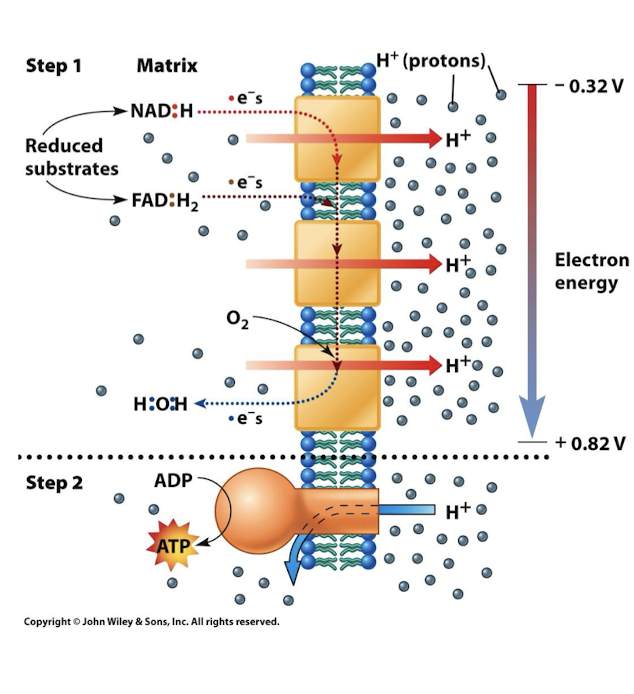
Energy for ATP provided by electrons in carriers NADH and FADH2
3 ATP per NADH
2 ATP per FADH2
Redox Potential (Eo)
A measure of the tendency of a chemical species to acquire electrons from a specific donor. Measured in volts; the more positive Redox potential, the stronger the affinity for electrons.
Electrons pass through the ETC from one acceptor to another, in each step they move to a molecule with greater ReDox potential, ultimately to O2.
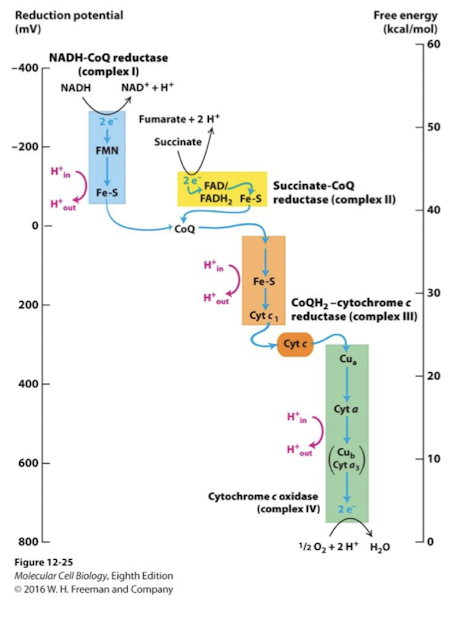
Electron Transport Chain
NADH and FADH2 donate their electrons to reduce the various electron carriers associated with four inner membrane protein complexes- collectively called the Electron Transport Chain
Electrons are passed through carriers with consecutively greater positive redox potential
Energy releasing reactions are coupled to conformational changes in three of the complexes
These changes pump protons (H+) across the inner membrane into the intermembrane space, establishing the proton force
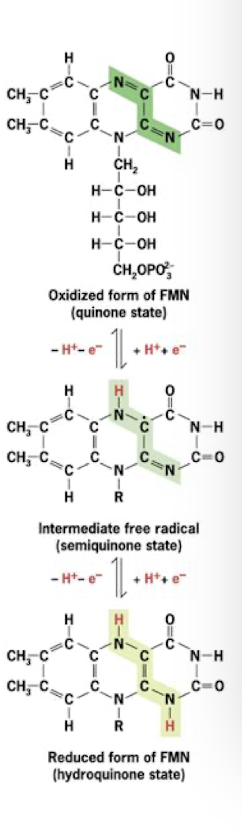
Flavoproteins
Electron Carrier in the Electron Transport Chain
Polypeptides containing 1 of 2 related prosthetic groups
FMN (flavin mononucleotide)
FAD (flavin adenine dinucleotide)
each accepts and donates 2 protons and 2 electrons
FMN in complex I
FAD in complex II (recall this is the electron carrier reduced by sucinate dehydrogenase in the TCAC)
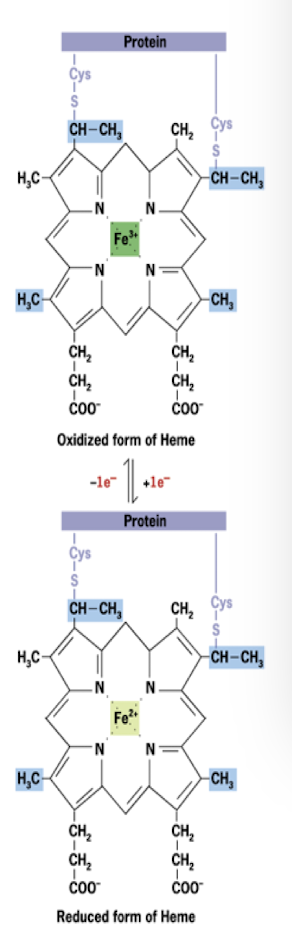
Cytochromes
Electron Carrier in the Electron Transport Chain
Redox proteins with heme prosthetic groups
Iron centers of heme alternate between Fe2+ and Fe3+ by gain or loss of single electron
3 distinct cytochromes in complexes III and IV
Another is associated with a peripheral membrane protein that ferries electrons between 2 of the 4 ETC complexes.
Three Copper Atoms
Electron Carrier in the Electron Transport Chain
All associated with complex IV
Accept and donate one electron at a time as they alternate between Cu2+ and Cu1+
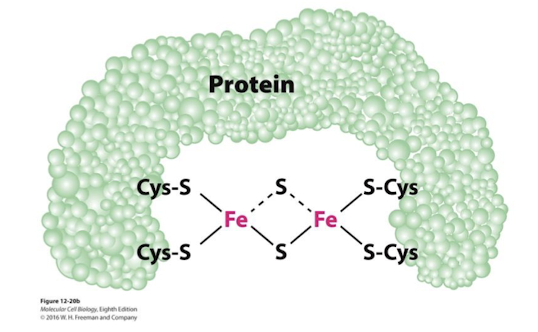
Iron-Sulfer Proteins
Electron Carrier in the Electron Transport Chain
Iron linked to non-heme sulfer centers in I, II, and III
Also accept and donate one electron at a time
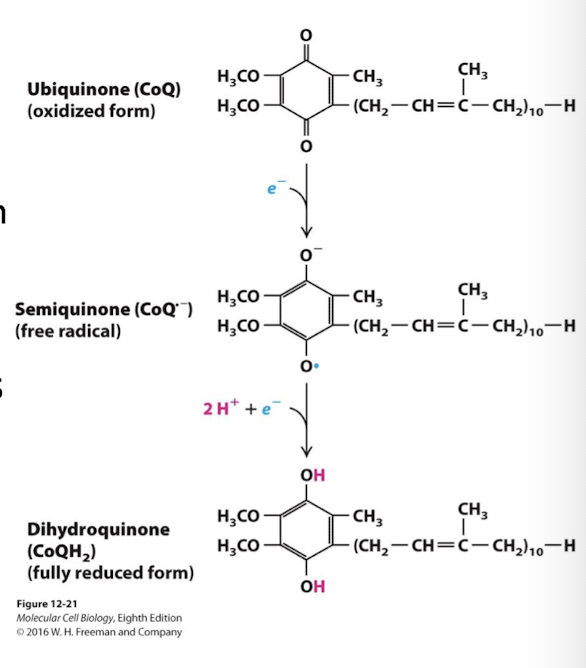
Ubiquinone (coenzyme Q: CoQ)
Electron Carrier in the Electron Transport Chain
Doesn’t have prosthetic group
It is lipid soluble due to a long hydrophobic tail
Capable of rapid lateral diffusion in the membrane leaflet
Accepts and donates 2 electrons and 2 protons
Partially reduced is a free radical = semiquinone (CoQH)
Fully reduced= dihydroquinone (CoQh2)
Lipid soluble, mobile electron carrier
Semi-reduced (1 electron)- semiquinone (CoQH)
Free-radical and highly reactive and therefore toxic to cells. Must be quickly converted to CoQ or CoQh2
Fully reduced (2 electrons)- dihydroquinone (CoQH2)
As CoQH2 it diffuses through the outer leaflet of the inner membrane from complexes I and II to complex III (CoQH2 cytochrome c reductase)
Prosthetic Goups
non-protein molecules, strongly, but usually non-covalently, bound to the protein complexes.
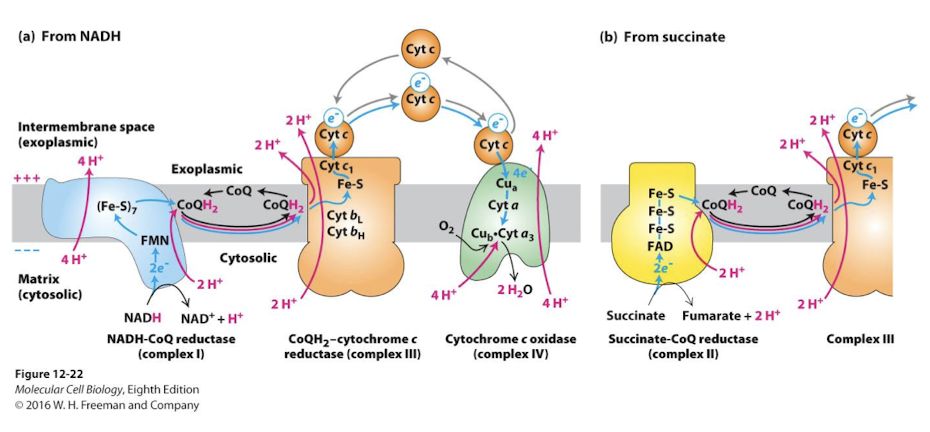
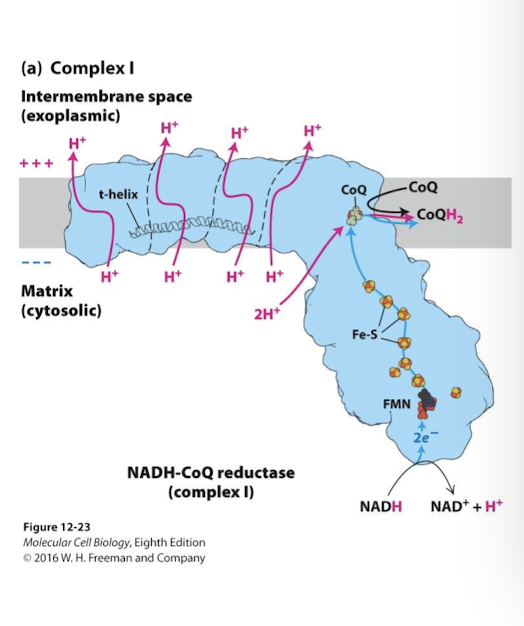
Complex I Electron Transport Chain
NADH-CoQ Reductase
Principle Function: Catalyze oxidation of NADH and reduction of ubiquinone (CoQ)
All electron carriers are in the soluble domain that extends into the matrix
Electrons are transferred from NADH to FMN. Then through seven Fe-S centers.
Final Step: the electrons reduce ubiquinone (CoQ) to dihydroquinone (CoQH2). 2 protons are absorbed from the matrix as CoQ is reduced
Bound to CoQH2 the complex changes conformation, shifting a horizontal helix- the t helix- that joins 4 sets of proton half-channels
This alters the iconic state of uniquely positioned residues within these channels that accept and release 4 protons into the inter-membrane space.
CoQH2 is now primed to deliver electrons to Complex III: CoQH2-Cytochrome c reductase
Several other enzymatic processes in the mitochondria (beta-oxidation for example) contribute to available CoQH2
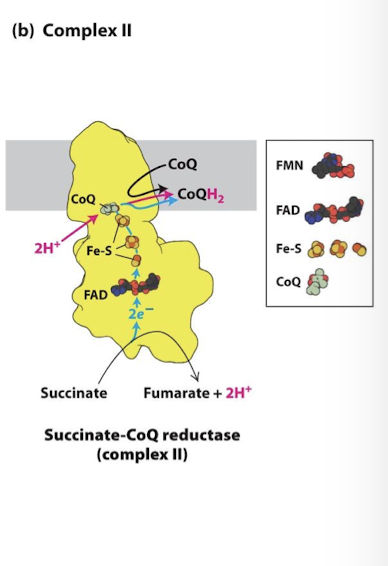
Complex II Electron Transport Chain
Succinate CoQ Reductase
The same complex of the Citric Acid Cycle that catalyzes reduction of FAD to FADH2
FAD is a prosthetic group in this complex
FADH2 remains physically associated with the complex and its electrons are passed through 3 Fe-S centers
There is no accompanying proton transfer
These electrons just like complex I also reduce CoQ to CoQh2
CoQH2 will deliver the electrons to complex III
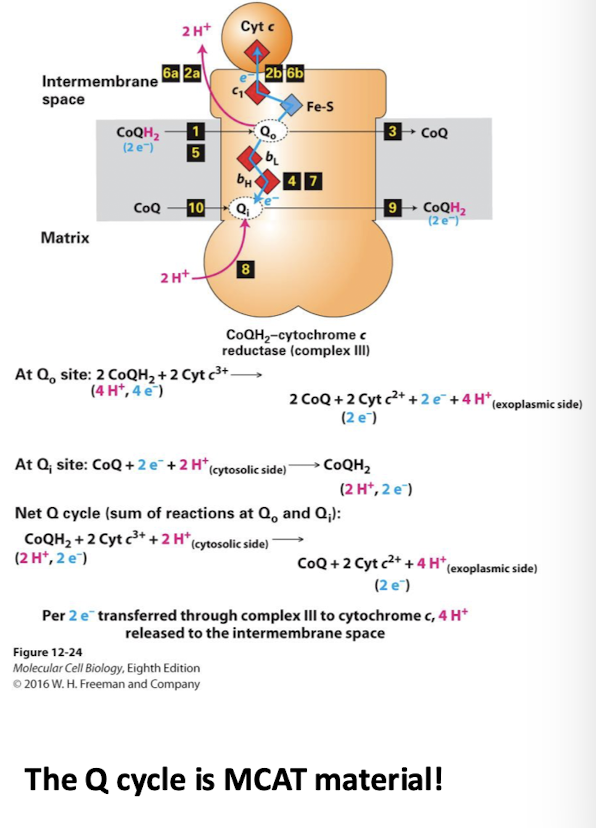
Complex III Electron Transport Chain
CoQH2-cytochrome c reductase
The Q cycle enhances the number of protons pumped into inter-membrane space
Oxidizes 2 CoQH2, but reduces one CoQ in the process
Only one CoQH2 equivalent is oxidized- meaning only one e- pair equivalent is consumed
For each CoQH2 that is oxidized two H+ are pumped into the inter-membrane space
So the net effect is the movement of a 4H+ into the inter-membrane space for the oxidation of one CoQH2 equivalent (one e- pair)
The Q0 site near the inter-membrane space binds a CoQH2
Its 2 H+ are released into the inter-membrane space
One electron immediately reduces the Cytochrome c peripheral membrane protein
The second electron passes through two cytochrome b carriers to a CoQ bound to the Qi in the inner leaflet
CoQ is released from the Q0 site
CoQH remains bound to the Qi site
A second CoQH2 binds Q0 and the process repeats
an e- reduced Cyt c PMP
2H+ are released into the inter-membrane space
The CoQH in Ai is fully reduced. It accepts two H+ from the matrix and is released into the inner leaflet
Results of the Q cycle per electron pair:
4 protons pumped into inter-membrane space
Only 1 CoQH2 equivalent is oxidized
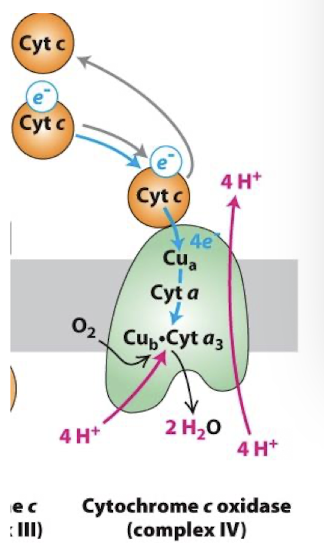
Cytochrome c peripheral protein
associated with the leaflet facing the inter-membrane space and is a mobile carrier between complex III and IV
It carrier 1 electron at a time to the final complex
Complex IV Electron Transport Chain
Cytochrome c oxidase
It takes 4 e- to reduce one O2- this equivalent to 2 e- pairs from either NADH or FADH2
Therefore, when considering how many protons are transported into the inter-membrane space per NADH and FADH2 we count 2 protons pumped by complex IV
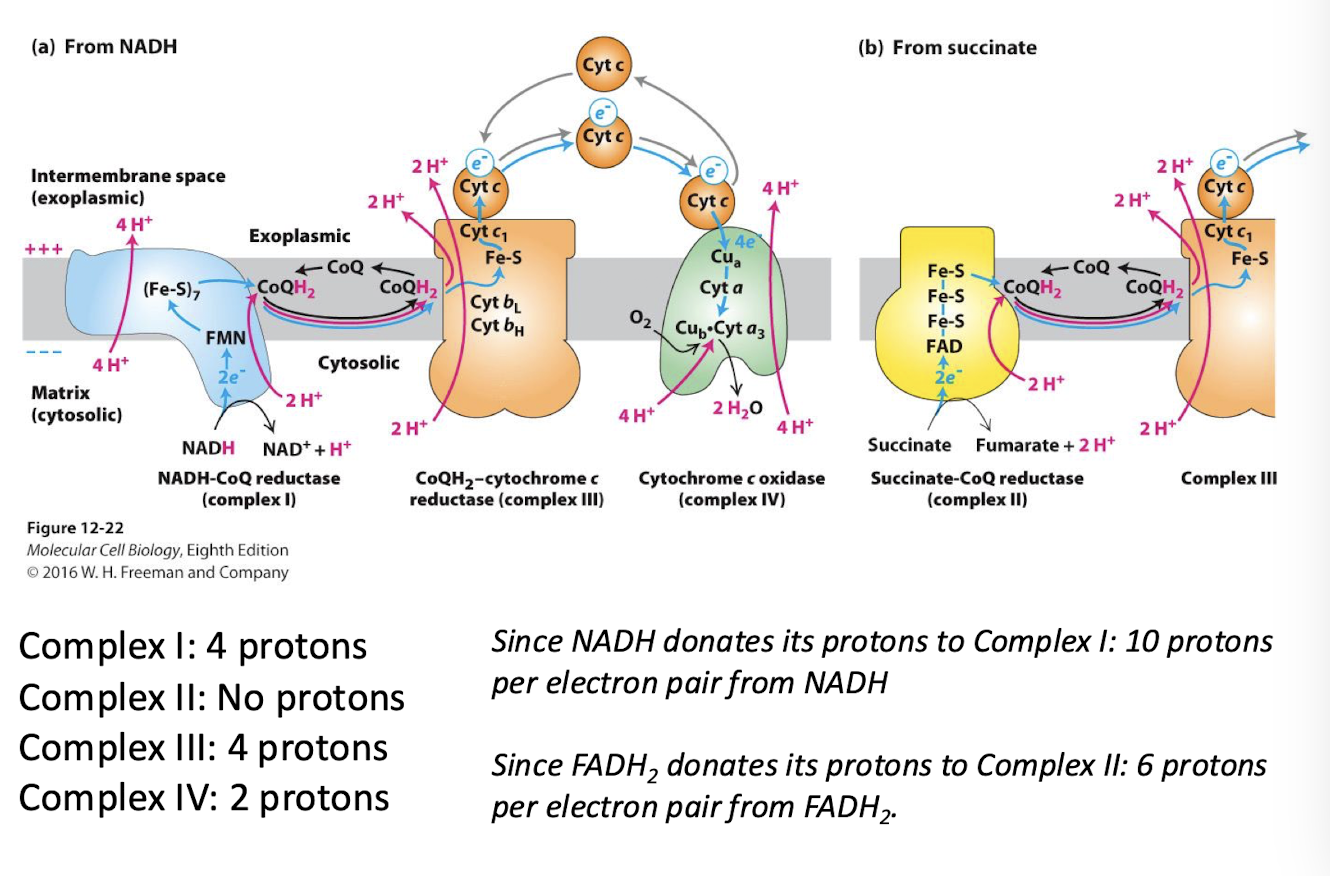
The Role of Mitochondria in the formation of ATP
Electron-Transport Complexes in descending order of reduction potential:
NADH-CoQ reductase (Complex I): Catalyzes transfer of electron pair from NADH to ubiquinone and translocates four H+ per pair
Succinate-CoQ reductase (Complex II): Catalyzes transfer of electron pair fro succinate to FAD to ubiquinone without translocation of H+
Co-QH2 cytochrome c reductase (Complex III): Catalyzes the transfer of electrons from ubiquinone to Cytochrome c peripheral membrane protein and translocates four H+ per pair
Cytochrome c oxidase (complex IV): catalyzes transfer of 4 electrons to O2 and translocates two H+ across the inner membrane per electron pair from NADH of FADH2
Reactive oxygen species are biproducts of electron transport
Superoxide (O2-) 1-2% of metabolized O2 is not converted to water but is instead partially reduced to form this toxic free radical
Free radicals: unstable ions with unpaired electrons
formed during normal metabolism
radicals and some non-radicals like H2O2 are highly reactive- can damage macromolecules such as DNA by stealing or donating electrons
play significant role in aging and disease
many sources in animal cells, but the major source is the electron transport chain
Superoxide dismutase (SOD)
An enzyme that converts O2- into hydrogen peroxide (H2O2) which is then converted to water by other enzymes
protects cells from damage from the superoxide radical
Overexpressing SOD in lab mice extends their life by 20%
Mammals have 3 version: cytoplasmic, extracellular, and mitochondrial
Loss of mitochondrial version leads to early leathality
Mutations in the cytoplasmic version are associated with familial amyotrophic lateral sclerosis
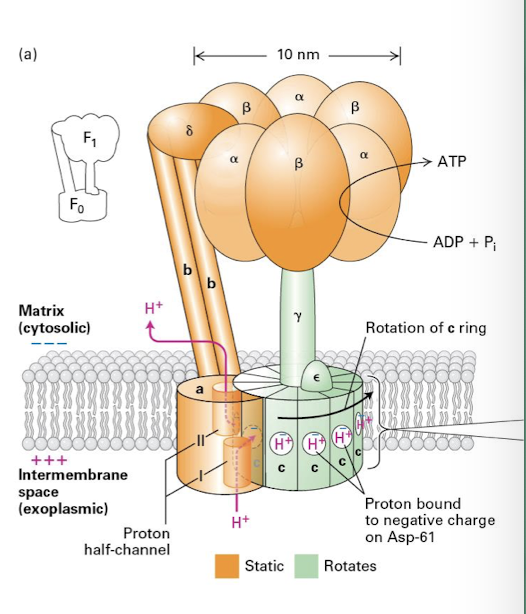
ATP Synthase
F-type Pumps Major Components
gama-subunit has a club shaped head that rotates inside the catalytic head composed of 3 alpha 3 beta subunits
2 beta subunit project into the matrix and hold the rotating head of the complex in place
The rotation of the gama-subunit within the head promotes conformational changes in the catalytic beta subunits that drives the phosphorylation reactions
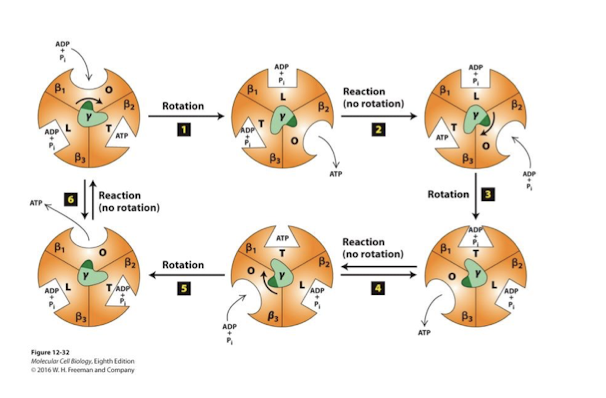
Binding Change Mechanism
Change in conformation and affinities for ATP synthesis
Catalytic head contains 3 pairs of alpha/beta subunits- the beta subunits possess catalytic sites for ADP phosphorylation.
beta subunit’s 3 distinct conformations allows:
capture of ADP/Pi release ATP Loose
catalysis of the reaction Tight
release of ATP Open
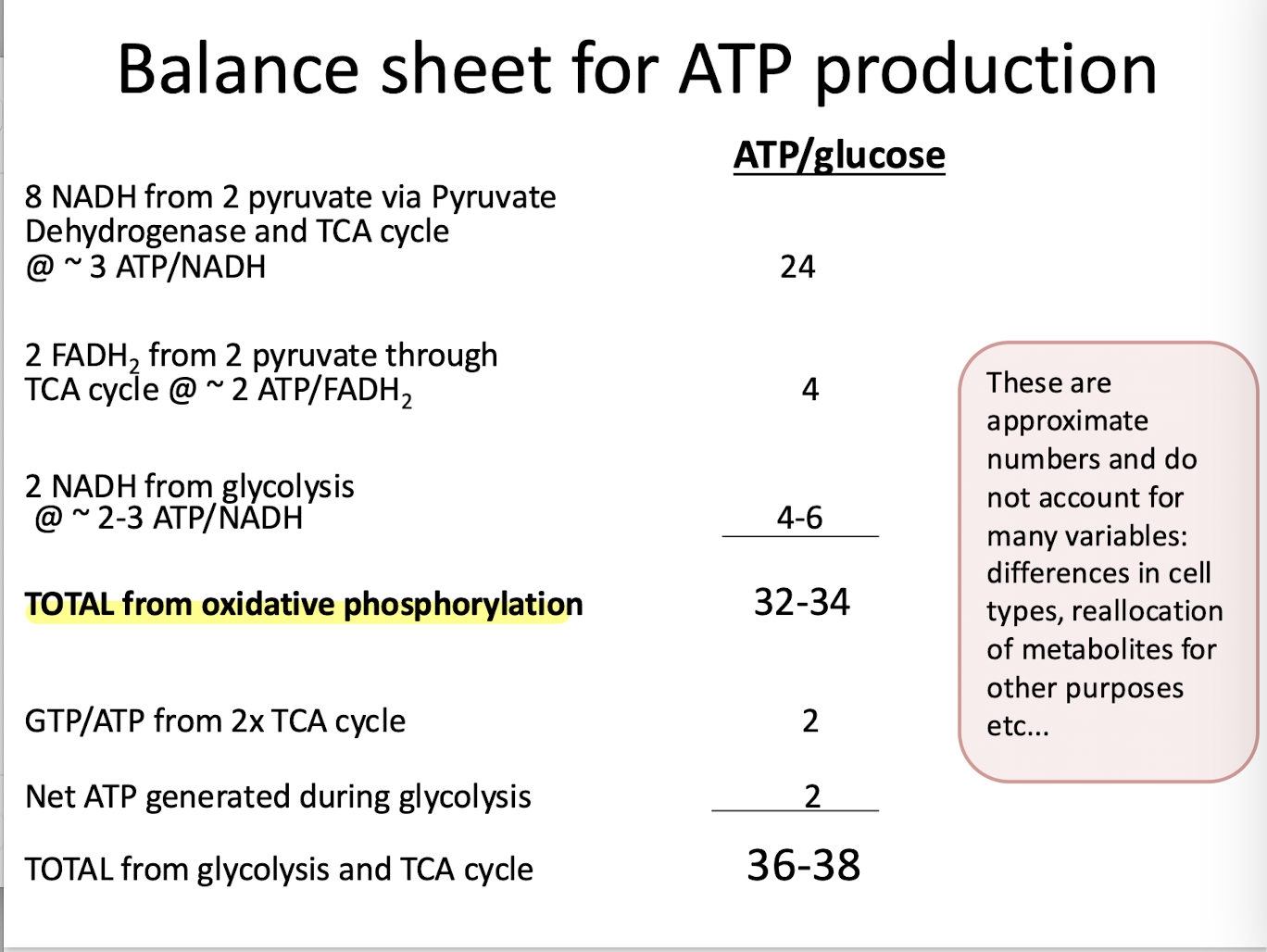
Balance Sheet for ATP production