Photosynthesis: Chlorophyll Extraction and Absorption Spectrum
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Purpose of the lab
-chlorophyll absorbs packets of light/energy(photons)
-photons are associated with wavelength (measure of energy)
-450 nm > than 650nm
*Chlorophyll absorbs 450 nm/650nm well
-photons allow the electrons to be removed from the electron to produce oxygen(produce ATP)
Absorption spectrum
-pattern of absorption by a substance
How a spectrophotometer functions
-operates from 400-700 nm(wavelength)
white light enters the spectrometer
white light is split by monochromator(split into individual wavelength (# wavelength=color)
monochromator selects a wavelength and passes through solution
light not absorbed converts into a electrical signal( processes as a number)
We are analyzing..
Derived from Spinach leaves
carotenoids, chlorophyll a, chlorophyll b
1st: Chlorophyll is separated from carotenoids
2nd: Chlorophyll a is separated from chlorophyll b
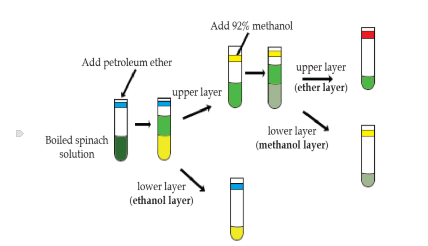
How were the pigments(carotenoids) separated from Spinach Leaves
boiled spinach leaves in water(soften the cell walls)
boiled spinach in ethanol(to extract the cell walls)
How were Chlorophylls separated from Carotenoids
petroleum was added to the ethanol extract
inverted it
allowed for the ethanol and petroleum to separate in two layers
ethanol: lower layer, yellow due to carotene(placed in the blue tube
petroleum ether: upper layer, green due to chlorophyll
add methanol to another tube and extract the chlorophyll a+b
Red tube(petrolume) chlorophyll a
Yellow tube(methonal) chlorophyll b
What separated Carotene
ethanol
What separated chlorophyll B
methanol
What separated chlorophyll A
petroleum ether
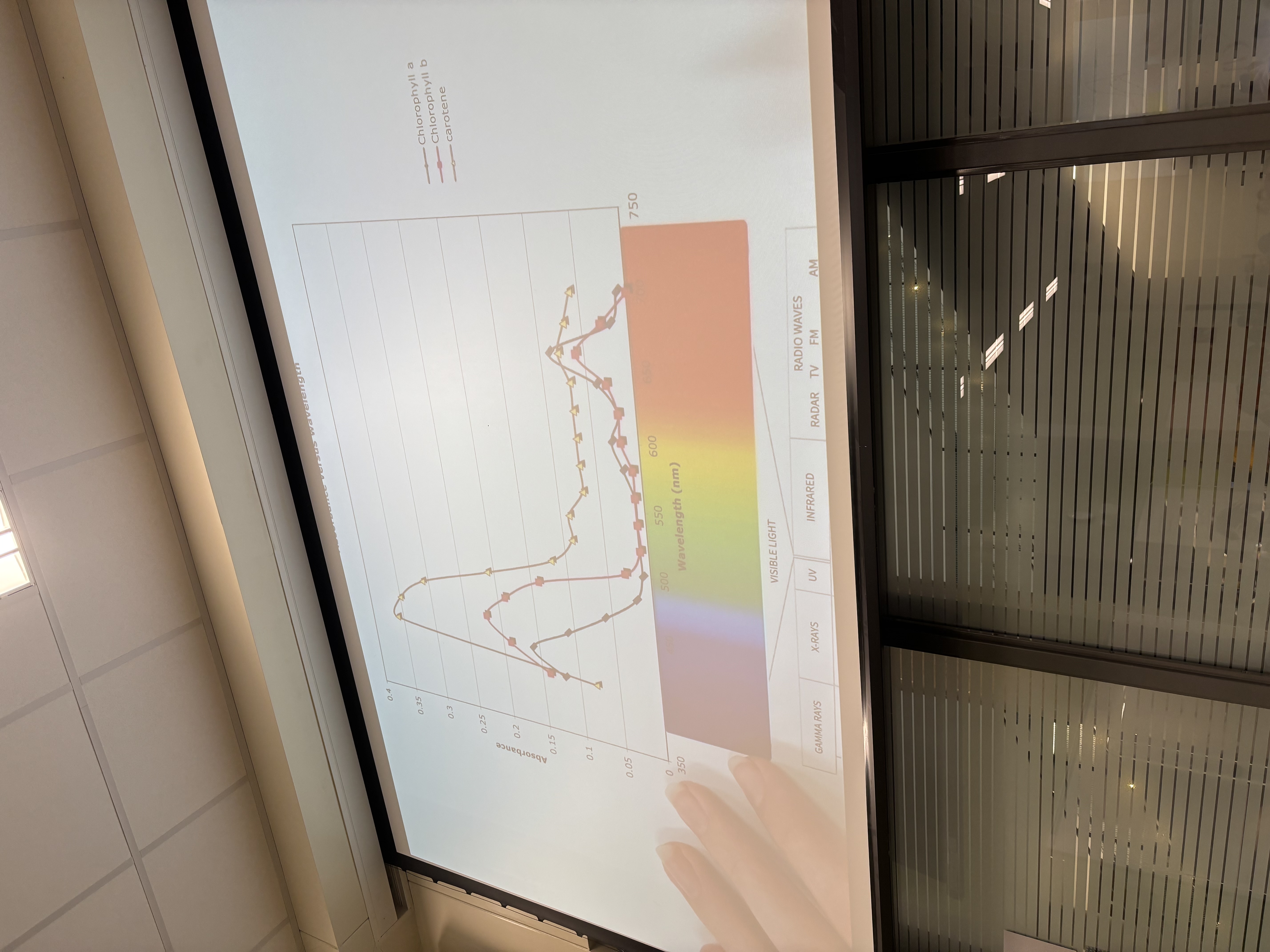
What was absorbed about the graph from Chlorophyll A+B and carotene
*chlorophyll A/B have two peaks at 450(Purple/blue) and 650(red/orange)
*carotene peaks at one point around 450(purple/blue)
Observation of Original/diluted chlorophyll A+B
the absorbance was greater in the original substance
*Concentration of the pigment was higher
-lower concentration means lower absorbance peak
-peaks will still occur in the same area
Does changing the pigment concentration alter the position(wavelength) of the peaks?
-does not alter
-the peak: represents where color is absorbed the most
*changing the concentration would allow for more light to be absorbed
Does changing the concentration of the pigment alter the height(absorbance) of the peak?
-absorbance(height) would change there are more molecules to absorb more light
-peak would increase if concentration increases
the relationship between concentration of pigment and height of pigment
-the more concentration the more absorbance
what would be more helpful in identifying a substance(position or height)
-position of the peak( wavelength) correlates to how much light a substance can absorb (determined by the molecule composition)
-height(absorbance) correlated to how much there is
Why are plants green
they can’t absorb green(green is reflected)
-only absorb blue and red
How to calculate Energy
2 × 10^-16/wavelength(nanometers)
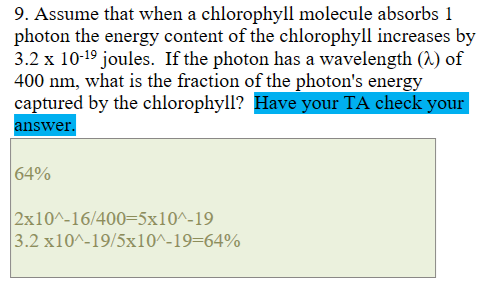
sample problem
for observation