Physics Chapter 9: Kinetic Theory of Gases
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
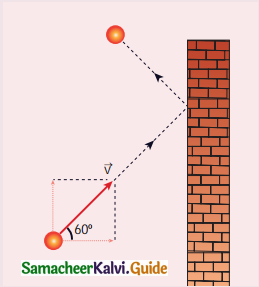
A particle of mass m is moving with speed u in a direction which makes 60° with respect to x-axis. It undergoes elastic collision with the wall. What is the change in momentum in x and y direction?
(a) ∆px = – mu, ∆py = 0
A sample of ideal gas is at equilibrium. Which of the following quantity is zero?
(c) average velocity
An ideal gas is maintained at constant pressure. If the temperature of an ideal gas increases from 100K to 1000K then the rms speed of the gas molecules:
(b) increases by 10 times
Two identically sized rooms A and B are connected by an open door. If the room A is air conditioned such that its temperature is 4° lesser than room B, which room has more air in it?
(a) Room A
The average translational kinetic energy of gas molecules depends on:
(a) number of moles and T
If the internal energy of an ideal gas U and volume V are doubled then the pressure:
b) remains same
The ratio γ = CP/CV for a gas mixture consisting of 8 g of helium and 16 g of oxygen is:
(c) 27/11
A container has one mole of monoatomic ideal gas. Each molecule has f degrees of freedom. What is the ratio of γ = CP/CV?
(d) f+2/f
If the temperature and pressure of a gas is doubled the mean free’ path of the gas molecules
(a) remains same
Which of the following shows the correct relationship between the pressure and density of an ideal gas at constant temperature?
A sample of gas consists of μ1 moles of monoatomic molecules, μ2 moles of diatomic molecules and μ3 moles of linear triatomic molecules. The gas is kept at high temperature. What is the total number of degrees of freedom?
(a) [3μ1 + 7(μ2 + μ3)] NA
If sp and sv denote the specific heats of nitrogen gas per unit mass at constant pressure and constant volume respectively, then:
(b) sp and sv = R/28
Which of the following gases will have least rms speed at a given temperature?
(d) Carbon dioxide
For a given gas molecule at a fixed temperature, the area under the Maxwell- Boltzmann distribution curve is equal to
a) PV/kT
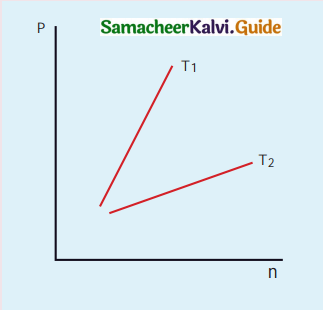
The following graph represents the pressure versus number density for ideal gas at two different temperatures T1 and T2. The graph implies:
(b) T1 > T2
Why moon has no atmosphere?
The escape speed of gases on the surface of Moon is much less than the root mean square speeds of gases due to low gravity. Hence all molecules of the gases escape from the surface of the Moon easily.
What is the relation between the average kinetic energy and pressure?
P = 23KE¯¯¯¯¯¯¯¯
Define the term degrees of freedom
The minimum number of independent coordinates needed to specify the position and configuration of a thermo-dynamical system in space is called the degree of freedom of the system.
State the law of equipartition of energy.
According to kinetic theory, the average kinetic energy of a system of molecules in thermal equilibrium at temperature T is uniformly distributed to all degrees of freedom (x or y or z directions of motion) so that each degree of freedom will get 1/2 kT of energy. This is called law of equipartition of energy.
Deduce Charle’s law based on kinetic theory.
PV = 2/3U
For a fixed pressure, the volume of the gas is proportional to the internal energy of the gas.
(or)
Average kinetic energy is directly proportional to absolute temperature. It is implied that,
V ∝ T
(or) V/T = constant.
Deduce Boyle’s law based on kinetic theory
we know that PV = 2/3 U
But the internal energy of an ideal gas is equal to N times the average kinetic energy (∈) of
each molecule.
U = N∈
For a fixed temperature, the average translational kinetic energy ∈ will remain
constant. It implies that
PV = 2/3 N∈ Thus PV = constant
Therefore, pressure of a given gas is inversely proportional to its volume provided the temperature remains constant.
Deduce Avogadro’s law based on kinetic theory.
This law states that at constant temperature and pressure, equal volumes of all gases
contain the same number of molecules. For two different gases at the same temperature
and pressure, according to kinetic theory of gases,
List the factors affecting the mean free path.
Mean free path increases with increasing temperature. As the temperature increases, the average speed of each molecule will increase. It is the reason why the smell of hot sizzling food reaches several metre away than smell of cold food.
The mean free path increases with decreasing pressure of the gas and diameter of the gas molecules.
What is the reason for the Brownian motion?
According to kinetic theory, any particle suspended in a liquid or gas is continuously bombarded from all directions so that the mean free path is almost negligible. This leads to the motion of the particles in a random and zig-zag manner