IB Biology SL 2025 Exam - Unit 1 Study Guide
1/74
Earn XP
Description and Tags
Covering topics from Unit 1 that could be on the 2025 test!
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
How is water the medium of life?
It is thought that the first cells came from water, specifically oceans. During formation of first cells, a small batch of water was enclosed in a membrane, allowing for substances to dissolve and chemical reactions to occur, which allows the processes of life to occur
Why does water have a polarity?
Within a water molecule, there is an unequal sharing of electrons (more on the oxygen than hydrogen), causing one side to be more “negatively charged” and the other “positively charged”
How does water stick together through cohesion?
Water sticks together because of the mutual attractions between the water molecules (caused by hydrogen bonds)
How does water stick to surfaces through adhesion?
Hydrogen bonds form between the water molecules and surfaces of solids composed of polar molecules, allowing them to stick together
How are the solvent properties of water linked to its role as a medium for metabolism and for transport in plants and animals?
The polar nature forms shells around charged and polar molecules, preventing them from clumping together and remaining in solution
In metabolism, substances are dissolved in the cytoplasm. Dissolved enzymes catalyze metabolic reactions, and the solutes move around and interact
In transport, xylem and phloem sap transport mineral ions and sucrose respectively to the plant. In animals, blood transports glucose, amino acids, oxygen, and fat molecules (coated in phospholipids to prevent contact between water and fat)
What are the Physical Properties of Water to consider?
Buoyancy, Viscosity, Thermal Conductivity, and Specific Heat
Black-throated loon (Gavia arctica) and its interaction with water
Since the loon flies more often than it swims, it requires more aerodynamic wings to fly efficiently, while its feet are not that strong. Additionally, since air has a much lower thermal conductivity, it can maintain its body temperature easier
Ringed Seal (Pusa Hispida) and its interaction with water
Since the seal swims more, it requires strong flippers to be able to move through a more viscous medium. However, since water has a higher specific heat capacity, it provides a more stable thermal environment despite water having higher thermal conductivity
Chemical Properties of Carbon Atoms
Can form up to 4 covalent bonds with elements such as H, O, N or P, and these covalent bonds can chain up to any length. The covalent bonds can spread out as far to make a tetrahedral shape
Sometimes, carbon’s bond angles can form rings, whether it’s all carbon or with other elements
Polysaccharides
A chain of monosaccharides
Polypeptides
A chain of amino acids
Nucleic Acids
A chain of nucleotides
Digestion of Polymers into Monomers via Hydrolysis
Polymers are deconstructed so that monomers can be used to build new polymers or as a source of energy
Polysaccharides deconstruct into monosaccharides
Polypeptides deconstruct into amino acids
Nucleic Acids deconstruct into nucleotides
Form & Function of Monosaccharides
Between 3-7 carbon atoms
Pentoses have 5, Hexoses have 6. Typically they’ll also have one oxygen in the carbon ring
Monosaccharides can connect to other monosaccharides to create polysaccharides. Glucose is a well-known one
Polysaccharides as Energy Storage Compounds
Branched structures of starch and glycogen allow them to be relatively compact despite their large mass, storing large amounts of energy without taking up much space
Structure of Cellulose
Composed of beta-glucose (typically more than 10,000 glucose subunits), and is only linked by 1-4 glycosidic bonds
Since they are not branched, they can only connect if it’s a pattern of regular-inverted
These form straight chains called microfibrils, which have very high tensile strength
Glycoproteins in cell-cell Recognition
Composed of polypeptides with carbohydrate attached (typically an oligosaccharide, which is a monosaccharide linked with glycosidic bonds)
The carbohydrate is the part that is displayed, allowing cells to recognize other cells
Lipids
Diverse group of substances that dissolve in non-polar solvents, such as ethanol, toluene and propanone
Common groups include oils, fats, waxes, and steroids
Triglycerides
Made by combining 3 fatty acids and one glycerol
Each of the fatty acids are linked via condensation reactions, so 3 water molecules are produced. Since the hydrophilic parts are used up during this reaction, triglycerides are hydrophobic
Depending on the type of fatty acids, triglycerides can be fats or oils
Phospholipids
Similar to triglycerides, but there are two fatty acids linked to the glycerol, with the third group instead being a phosphate group
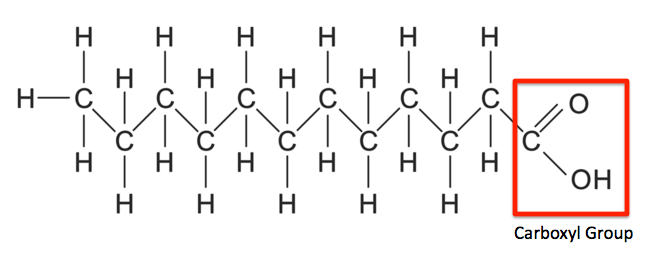
Saturated Fatty Acids
Fatty acids that have all single covalent bonds between the carbon atoms, allowing it to contain as much hydrogen as possible
Monounsaturated Fatty Acids
Fatty acids that have one double covalent bond amongst the carbon atoms
Polyunsaturated Fatty Acids
Fatty acids that have more than one double covalent bond amongst the carbon atoms
How are triglycerides used in adipose tissue for energy storage and thermal insulation?
They are chemically stable, so they don’t lose energy
They are immiscible in water, so they naturally form droplets in the cytoplasm and don’t have effects on the osmosis
They release twice as much energy per gram in cell respiration as carbohydrate
They can be used as insulators
They are liquid at room temperature, acting as shock absorbers
Formation of Phospholipid Bilayers
The hydrocarbon tails of phospholipids are attracted more to each other because they are hydrophobic, causing the “tails” to attract each other while the heads face the opposite directions
They form the basis of cell structures
Amphipathic
When part of a molecule is hydrophobic and another part is hydrophilic
Examples of non-polar molecules to pass through phospholipid bilayer
Steroids are the most common that can pass through phospholipid bilayers
Steroids are composed of 4 fused rings of carbon atoms (3 cyclohexane and 1 cyclopentane giving a total of 17 C atoms)
Their hydrophobic properties allow them to pass through phospholipid bilayers
Structure of Amino Acid
Central carbon atom called the alpha carbon, with 4 single covalent bonds
One is to a nitrogen atom of an amine group.
The second is to a carbon atom of a carboxyl group (-COOH)
A third goes to another hydrogen atom
A fourth links it to a side chain called an R group
Condensation reactions forming dipeptides and longer chains of amino acids
Dipeptides are formed with two amino acids and a condensation reaction. Polypeptides are formed with more than two
These are formed via peptide bonds
Dietary Requirements for Amino Acids
20 different amino acids are used by ribosomes to make polypeptides (plants make, animals obtain)
9 of 20 are essential in humans, this can be obtained by eating animal-based foods such as fish, meat, milk or eggs
How Many Combinations are Possible for Amino Acids?
For a polypeptide with n amino acids, there are 20n possibilities
Effect of pH and temperature on Protein Structures
High temperatures causes vibrations within the molecule that can break intermolecular bonds or interactions, changing the shape of the protein. Proteins vary in their heat tolerance
Acidic and alkaline pHs can cause the positive and negative charges on the R-group to change, breaking ionic bonds or causing new ones to form.
Denaturation
When the bonds and interactions are broken between molecules that cause the protein to not be able to return to its original state
What is DNA?
Deoxyribonucleic acid, a molecule that carries genetic code
Covalent bonds forms between the phosphate group and pentose sugar on each nucleotide to form a DNA backbone
Names of Nitrogenous Bases
C - Cytosine
T - Thymine
G - Guanine
A - Adenine (only in DNA)
U - Uracil (only in RNA)
Complementary Base Pairing as A Role For Double Helix Strands
Adenine pairs with Thymine (Uracil in RNA)
Cytosine pairs with Guanine
Since the nucleotides run antiparallel, one must be inverted in order to be able to form hydrogen bonds, so they adopt a helical shape
Differences between DNA & RNA
DNA has two polymers, RNA has one polymer
DNA is double-stranded, RNA is single-stranded
DNA has a deoxyribose pentose sugar, while RNA has a ribose pentose sugar
Complementary Base Pairing as A Role for Replicating Genetic Info
Since the nitrogenous bases can only pair with one other base, it allows an exact copy to be made during replication for DNA or transcription for RNA
Diversity of DNA
There are 4n possibilities of bases depending on how long the sequence is
Additionally, DNA molecules can be any length, adding to the potential diversity
Conservation of genetic code across all life forms
Through the use of codons (sequence of three bases), organisms use 64 codons to code for amino acids in order to carry out life functions.
The incredible thing is, with a few minor exceptions, all living organisms and all viruses use the same genetic code
Hydrogen Bond
An intermolecular force that, in the context of water, forms when a slightly positive hydrogen atom in one polar molecule is attracted to a slightly negative oxygen atom of another polar molecule
Cohesion in Xylem
Allows the transport of water under tension in plants
Continuous columns of water that are drawn up generate tension between soil particles and water molecules
Water in xylem can withstand tensions because hydrogen bonds make it cohesive
Cohesion on Water Surfaces
Water molecules are much more attracted to each other than air molecules, creating an elastic membrane. This allows certain things to be able to float on water
Water striders walk on the surface of the water
Capillary Action in Plants
The process where water molecules “move” through surfaces thanks to its strong suction forces on polar areas
In plants, water adheres to the cellulose, so any wall that dries out is automatically rewetted
Buoyancy
The upward force that is exerted on an immersed object
Since living organisms have a density close to water, it allows them to live in water habitats, as they don’t need to use much energy to float
Viscosity
The stickiness of a fluid, which determines how easily it can flow
Pure water has a higher viscosity than organic solvents because hydrogen bonds cause internal friction
Seawater has a higher viscosity than freshwater because of the dissolved salts
Viscosity of air is about 50x smaller than that of water at the same temperature
Thermal Conductivity
The rate at which heat passes through a material
Water has a relatively high thermal conductivity. The high water content of blood allows it to carry heats to other parts of the body where it is generated to other parts that need more heat
Specific Heat
The heat required to raise the temperature of 1g of a material by 1C or 1K (kelvin)
Specific heat capacity of water is 4.18Jg-1K-1
Water has a high heat capacity because of the hydrogen bonds resisting molecular motion
Alpha-Glucose
Made of glycogen molecules in liver or muscle cells that are branched. They can contain up to 60,000
When drawing a diagram of these, the OH is on the top of the 4th carbon
Beta-Glucose
Cellulose molecules found in plant cell walls that are unbranched chains. They can contain 15,000 or more
When drawing a diagram of these, the H is on the top of the 4th carbon
Starch
Energy molecule used in plants composed of alpha-glucose. Can contain more than a hundred thousand glucose subunits
Amylose - one type that’s an unbranched chain linked by 1-4 glycosidic bonds, which form a helical chain
Amylopectin - same structure but some 1-6 glycosidic bonds to make it branched
Glycogen
Energy molecule used in animals composed of alpha-glucose
Linked by 1-4 glycosidic bonds and branched by 1-6 glycosidic bonds
Can contain tens of thousands of glucose subunits
Hydrophilic
Substances that are more attracted to water and therefore dissolve in water
Hydrophobic
Substances that are more attracted to other molecules and therefore do not dissolve in water
Oils
Type of lipid that has a melting point below 20C, so they solidify at low temperatures
Fats
Type of lipid that melts between 20C and 37C so they are solid at room temperature and liquid at body temperature
Waxes
Type of lipid that melts above 37C, so they liquify at high temperatures
Steroids
Type of lipid that is a molecule, typically known with a 4-ring structure
Ester Bonds
Formed when fatty acids link with the glycerol in the triglyceride. Forms when an acid links with the hydroxyl group (-OH) in an alcohol
Cis-Fatty Acids
When hydrogen atoms lie on the same side of the two double-bonded carbon atoms in unsaturated fatty acids
Since they all lie on the same side, there is a bend in the hydrocarbon chain at the double bond, so they cannot pack as tightly. So, cis-unsaturated fatty acids are typically oils
Trans-Fatty Acids
When hydrogen atoms are on opposite sides. Because of this, there is no bend in the hydrocarbon chain, allowing them to have a higher melting point
Peptide Bonds
The process where amino acids are linked by joining the carbon from the carboxyl group and the nitrogen from the amine group
Ribosomes catalyze this reaction in cells
Essential Amino Acids
An amino acid that cannot be synthesized in sufficient quantities by the organism so they must obtain from eating
Non-Essential Amino Acids
An amino acid that can be synthesized through metabolic pathways by the organism
Beta-Endorphin
Natural pain killer secreted by the pituitary gland that is a polypeptide composed of 31 amino acids
Insulin
Small protein that contains two short polypeptides, one with 21 amino acids and another with 30 amino acids
Alpha Amylase
The enzyme in saliva that starts the digestion of starch
Single polypeptide of 496 amino acids, with one chloride ion and one calcium ion associated
Titin
Part of the structure of the muscle
34,350 amino acids in humans, 35,213 amino acids in mice
Nucleotide
Consist of 3 parts: pentose sugar, phosphate group and a nitrogenous base
RNA
Ribonucleic Acid that is a single, unbranched polymer of nucleotides
Examples of Monosaccharides
Glucose, Fructose, Galactose
Examples of Disaccharides
Maltose, Lactose, Sucrose
Examples of Polysaccharides
Starches, Fibers, Glycogen
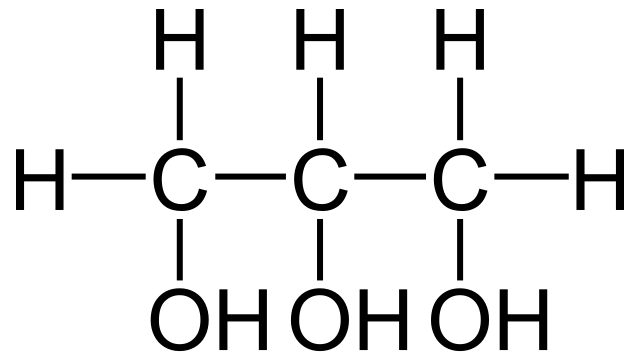
Glycerol Layout
Fatty Acid Layout