Periodic Table, Atomic Structure, and Chemical Bonding Review
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
79 Terms
What are the alkali metals on the periodic table?
Group 1 elements
What are the alkaline earth metals on the periodic table?
Group 2 elements
What are the transition metals on the periodic table?
Groups 3-11 elements
What are the halogens on the periodic table?
Group 17 elements
What are the noble gases on the periodic table?
Group 18 elements
Which element has the highest electronegativity?
Fluorine
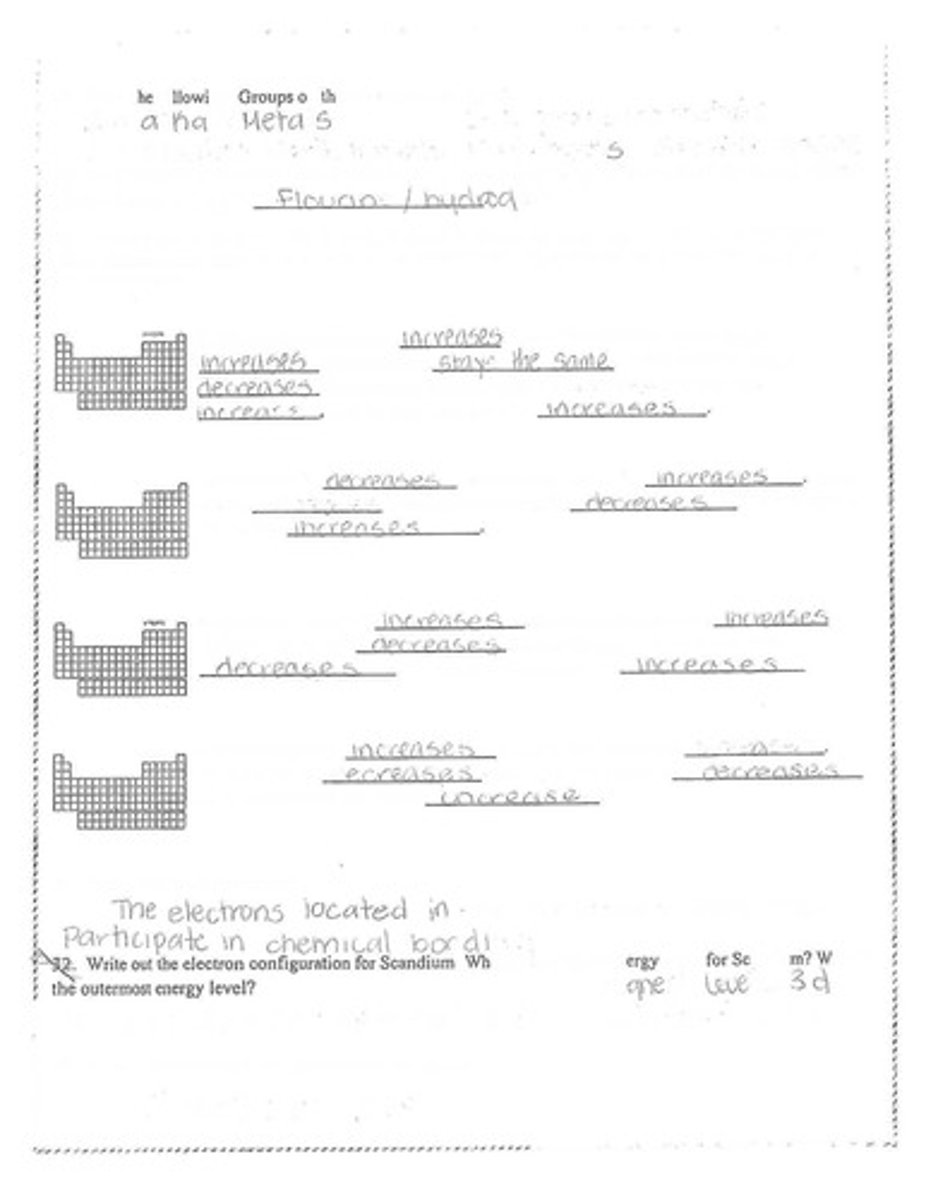
How does electronegativity change across a period?
It increases
How does electronegativity change down a group?
It decreases
What happens to effective nuclear charge across periods?
It increases
What happens to effective nuclear charge down a group?
It decreases
What happens to atomic radius across periods?
It decreases
What happens to atomic radius down a group?
It increases
What happens to ionization energy across periods?
It increases
What happens to ionization energy down a group?
It decreases
What are valence electrons?
Electrons located in the outermost shell that participate in chemical bonding
What is the electron configuration for Scandium?
[Ar] 4s² 3d³
What is the highest energy level for Scandium?
4
What is the outermost energy level for Scandium?
3
What is the noble gas configuration for Phosphorus?
[Ne] 3s² 3p³
What is chemistry?
The study of matter and its interactions.
What is a compound?
A substance formed from two or more elements chemically bonded together.
What is a mixture?
A combination of two or more substances that are not chemically bonded.
What is a heterogeneous solution?
A mixture where the components are not uniformly distributed.
What does VSEPR stand for?
Valence Shell Electron Pair Repulsion.
What is a chemical property?
A characteristic of a substance that can only be observed during a chemical reaction.
What is a physical property?
A characteristic of a substance that can be observed without changing its chemical composition.
What is an element?
A pure substance that cannot be broken down into simpler substances.
What is an isotope?
Atoms of the same element with different numbers of neutrons.
What is a homogeneous solution?
A mixture where the components are uniformly distributed.
What is the difference between a physical and chemical change?
Only a chemical change creates something new and different.
What are the shapes of the s, p, and d orbitals?
s is spherical, p is dumbbell-shaped, and d has complex shapes.
What type of reaction is MgCl2 + 2NaBr → 2NaCl + MgBr2?
Double replacement reaction.
What type of reaction is 2C2H2 + 5O2 → 4CO2 + 2H2O?
Combustion reaction.
What type of reaction is H2 + O2 → H2O?
Synthesis reaction.
What is the activity series used for?
To predict if a chemical change will happen in a reaction.
What is Rutherford's gold foil experiment?
An experiment that discovered the small, dense, positive nucleus of the atom.

What did Thompson discover using the cathode ray tube experiment?
The electron.

What is the definition of a proton?
A positively charged particle found in the nucleus of an atom.
What is the definition of a neutron?
A neutral particle found in the nucleus of an atom.
What is the definition of an electron?
A negatively charged particle found in the electron cloud surrounding the nucleus.
How are atoms identified?
By the number of protons they contain.
What is the Law of Conservation of Mass?
Matter cannot be created or destroyed.
What is the mass number?
The total number of protons and neutrons in an atom.
How many neutrons does Cl-35 (atomic number 17) have?
18 neutrons.
What is the difference between an electron in the ground state vs. excited state?
Electrons have lower energy in the ground state and higher energy in the excited state.
What is a photon?
A quantum of light.
What is the order of increasing energy for the following sublevels: 2s, 4s, 3d, 3p?
2s, 3p, 4s, 3d.
Who is credited with the arrangement of the modern periodic table?
Dmitri Mendeleev.
What is electronegativity?
The tendency of an atom to attract electrons in a chemical bond.
What is the trend for electronegativity across a period?
Electronegativity increases across a period.
What is the trend for atomic radius down a group?
Atomic radius increases down a group.
What is the trend for ionization energy across periods?
Ionization energy increases across periods.
What is the trend for effective nuclear charge across periods?
Effective nuclear charge increases across periods.
What is the formula for magnesium chloride?
MgCl₂.
What is the formula for Iron II chloride?
FeCl₂.
How many valence electrons does Nitrogen (atomic number 7) have?
5.
Does the bonding of two elements increase or decrease potential energy?
It decreases potential energy and increases stability.
What is unique about the electrons in a metallic bond?
They form a 'sea of electrons'.
What is the molar mass of CO?
28.0 g/mol.
How many moles are in 18 grams of NaCl?
.31 mol.
What is the Lewis structure for H₂S?
H-S-H (Bent shape).
What is the Lewis structure for NH₃?
H-N-H (Pyramidal shape).
What is the balanced equation for the reaction of barium chloride and potassium sulfate?
BaCl₂ + K₂SO₄ → BaSO₄ + 2KCl (double replacement).
What type of reaction occurs when sodium metal reacts with iron (III) chloride?
Single replacement.
What is the name of the compound BBr₃?
Boron tribromide.
What is the formula for calcium sulfate?
CaSO₄.
What is the formula for sodium bromide?
NaBr.
What is the formula for potassium acetate?
KC₂H₃O₂.
What is the formula for iron (II) phosphide?
Fe₃P₂.
What is the formula for titanium (IV) nitrate?
Ti(NO₃)₄.
What is the formula for disilicon hexabromide?
Si₂Br₆.
What is the formula for copper (I) phosphate?
Cu₃PO₄.
What is the formula for tetrasulfur dinitride?
S₄N₂.
What is the formula for carbon tetrachloride?
CCl₄.
What is the formula for vanadium (III) sulfide?
V₂S₃.
What is the formula for magnesium sulfate heptahydrate?
MgSO₄·7H₂O.
What is the formula for ammonium oxide?
NH₄₂O.
What is the formula for phosphorus?
P.
What is the formula for tin (IV) selenide?
SnSe₄.