3.1.6.1 chemical equilibria + Le Chatelier's principles
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
many chemical reactions are _____
reversible
what sign is indicate that the reaction is reversible?
⇌
→ represents the forward reaction
← represents the backwards reaction
where can equilibrium only be reached?
in a closed system
what is a closed system?
a system where the reactants + products cannot escape
a chemical equilibrium can only be achieved if what?
if no more reactants are added or removed from the reaction mixture
what happens if reactants are added or if products are removed?
then the equilibrium position will then shift
how do you know when equilibrium has been reached?
when the properties of the system (eg density, concentration, colour, pressure) do not change with time
outline dynamic equilibrium
forwards rate is equal to the backwards rate
concentrations remain constant (not equal)
for most reactions the effect of changing reaction conditions can be predicted using what?
Le Chatelier’s principle
define Le Chatelier’s principle
equilibrium will shift to oppose the change on a system
give 3 factors which affect the position of equilibrium
concentration
pressure
temperature
[X] means what?
concentration of X
what must happen if, at a fixed temperature, the concentration of any of the species involved in an equilibrium reaction is changed?
then the concentration of the other species must also change
explain what happens if the concentration of a reactant is increased
the system will shift to oppose this change + will reduce the concentration the concentration of the reactant by reacting some of it (+ so forming more product)
this shifts the equilibrium to the right hand side

explain what happens if the concentration of a product is increased
the system will shift to oppose this change + will reduce the concentration of the product by reacting some of it (+ so forming more reactants)
this shifts the equilibrium to the left hand side
when does pressure only affect the position of a chemical equilibrium?
when gases are involved
explain what happens if the total pressure is increased
equilibrium shifts to oppose the increase in pressure
so equilibrium will shift to the side of the reaction has fewer gaseous moles, to reduce the pressure

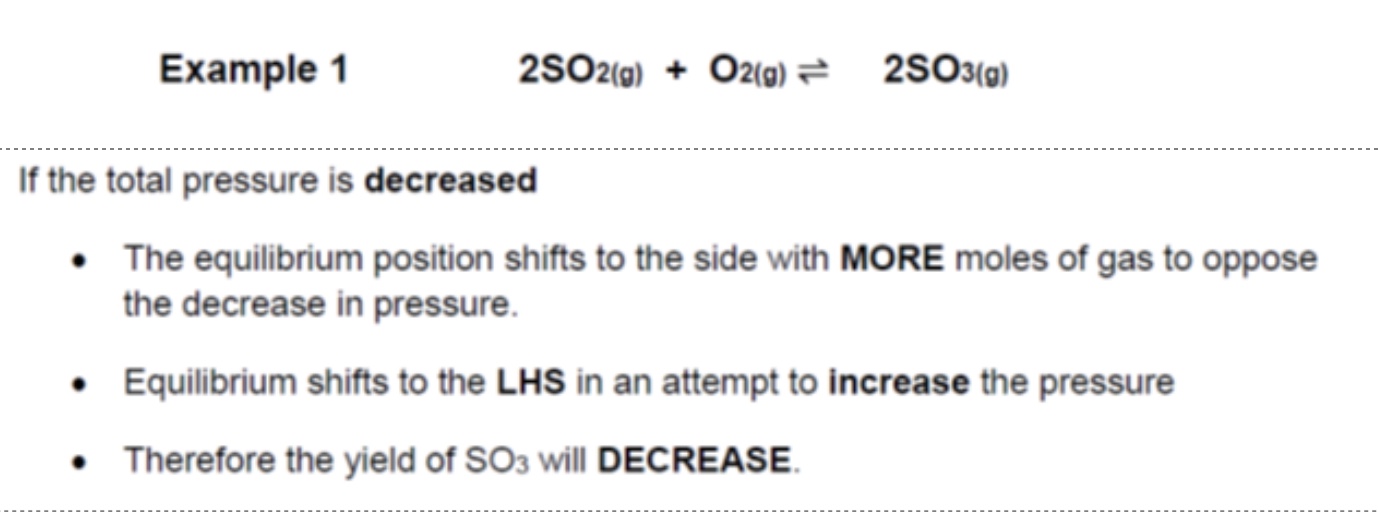
explain what happens if the total pressure is decreased
equilibrium shifts to oppose the decrease in pressure
so equilibrium will shift to the side of the reaction that has more gaseous moles, to increase the pressure
what happens if the pressure is increased b the number of moles of gas are the same in both sides of the equation?
altering the pressure would have no effect on the position of the equilibrium
instead the rates of the forward + backward reactions are increased equally

outline an exothermic reaction
△H is negative
the system gives out heat to the surroundings → increase in temp
outline an endothermic reaction
△H is positive
the system takes in heat from the surroundings → decrease in temp
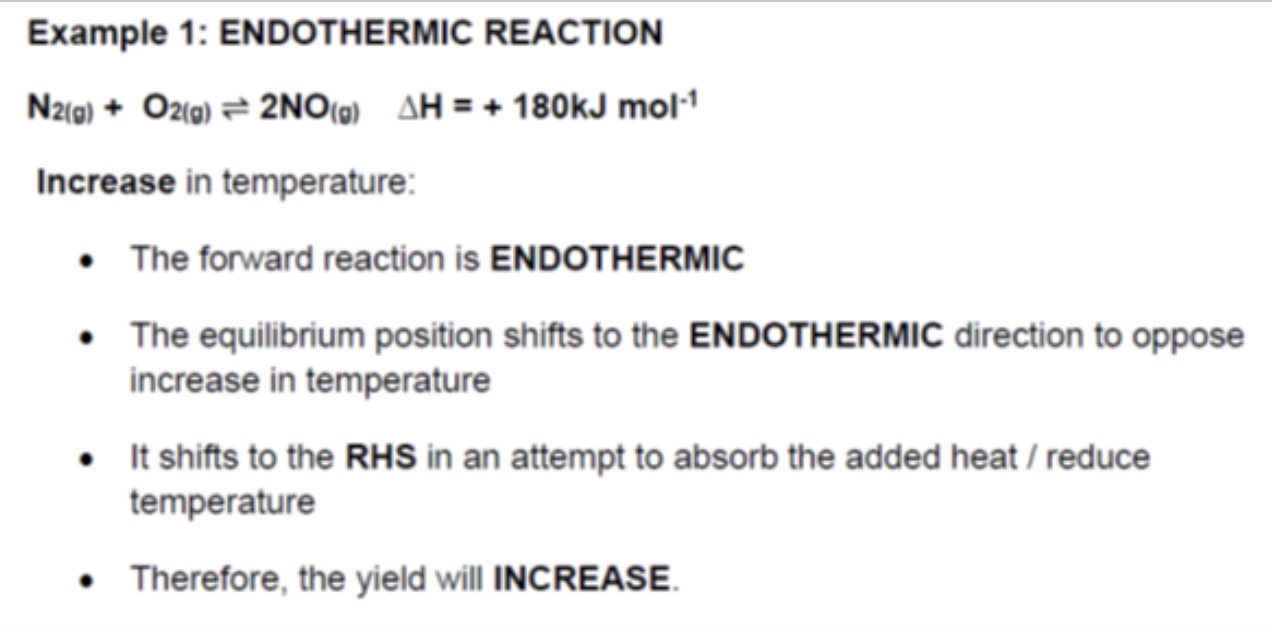
explain what happens if temperature is increased
the equilibrium will shift to oppose the increase in temperature
so equilibrium shifts in the endothermic direction to try + reduce the temperature by absorbing the added heat
explain what happens if the temperature is decreased
the equilibrium will shift to oppose the decrease in temperature
so equilibrium shifts in the exothermic direction to try to increase the temperature by giving out the added heat

outline the effect the addition of a catalyst has to an equilibrium mixture
it has no effect on the yield or position of equilibrium
the catalyst increases the rate of both forward + backward reactions equally
what do exam questions often ask about regarding equilibrium mixtures? how do you answer this?
the time taken to reach equilibrium (this refers to the rate of reaction)
answers should include the traditional collision theory ideas from kinetics, regardless of the position of equilibrium
give the structure of a Le Chatelier’s exam answer template
state which way the equilibrium will shift
state why

if equilibrium shifts to the right, yield ______. if equilibrium shifts to the _____, yield decreases.
increases
left
explain what chemists need to compromise on when producing chemicals in equilibrium reactions
its important they produce the best possible yield bearing in mind the cost + time involved
so the manufacturer aims for the highest possible yield, in the shortest possible time + for the lowest possible cost → they therefore need to compromise
example 1 — Haber process
outline this reaction + products
the synthesis of ammonia from nitrogen + hydrogen
an iron catalyst is used
ammonia is used in the production of fertilisers, synthetic fibres + plastics
explain why a compromise temperature is used for the Haber process
it is an exothermic process so the best equilibrium yield of ammonia is obtained at low temps → as this would move the equilibrium position to the right
however at low temps the rate of reaction is slow so a compromise temperature is used
explain why a compromise pressure is used for the Haber process
high pressures will result in a high yield of ammonia as increasing pressure moves the equilibrium to the side with fewer moles of gas
however high pressures are expensive to generate + complex equipment is required to withstand such pressures, which is also expensive
→ therefore a compromise pressure is used
what is the role of the catalyst in the Haber process?
it reduces the time taken for the reaction to reach equilibrium
what do compromise conditions give a balance between?
yield, rate + cost
example 2 - production of ethanol
outline this reaction + what ethanol is used for
main source of ethanol is from ethene from crude oil
a concentrated phosphoric catalyst is used
ethanol has many industrial uses eg making cosmetics, drugs + detergents
explain why a compromise temp is used for the production of ethanol
high temp = fast rate
low temp = good yield (equilibrium would move to right)
therefore a compromise temp is used to give a balance between rate + yield
explain why a compromise pressure is used for the production of ethanol
high pressure = fast rate + high yield
but high pressures are expensive to generate + need expensive, complex equipment
therefore compromise pressure is used to give a balance between rate, yield + cost