PHARM Hematology Agents Flashcards 💊 – IRAT 8
1/73
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
Anticoagulants:
Vitamin K Antagonists (e.g., Warfarin)
Direct Oral Anticoagulants (DOACs):
Direct Thrombin Inhibitors (e.g., Dabigatran)
Factor Xa Inhibitors (e.g., Rivaroxaban, Apixaban)
Heparins: Unfractionated Heparin (UFH), Low-Molecular-Weight Heparin (LMWH) (e.g., Enoxaparin), Parenteral Direct Thrombin Inhibitors (e.g., Argatroban, Bivalirudin)
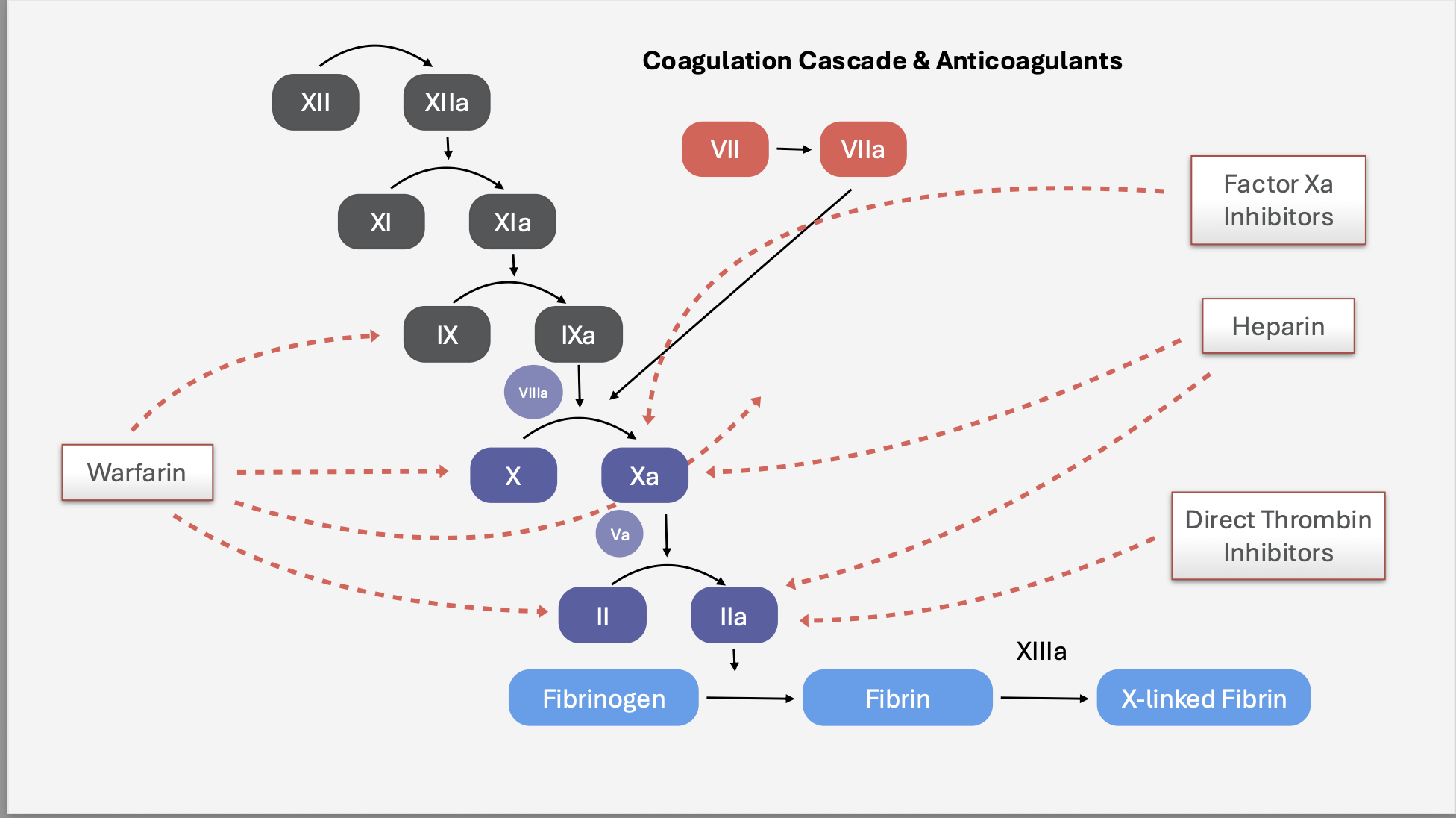
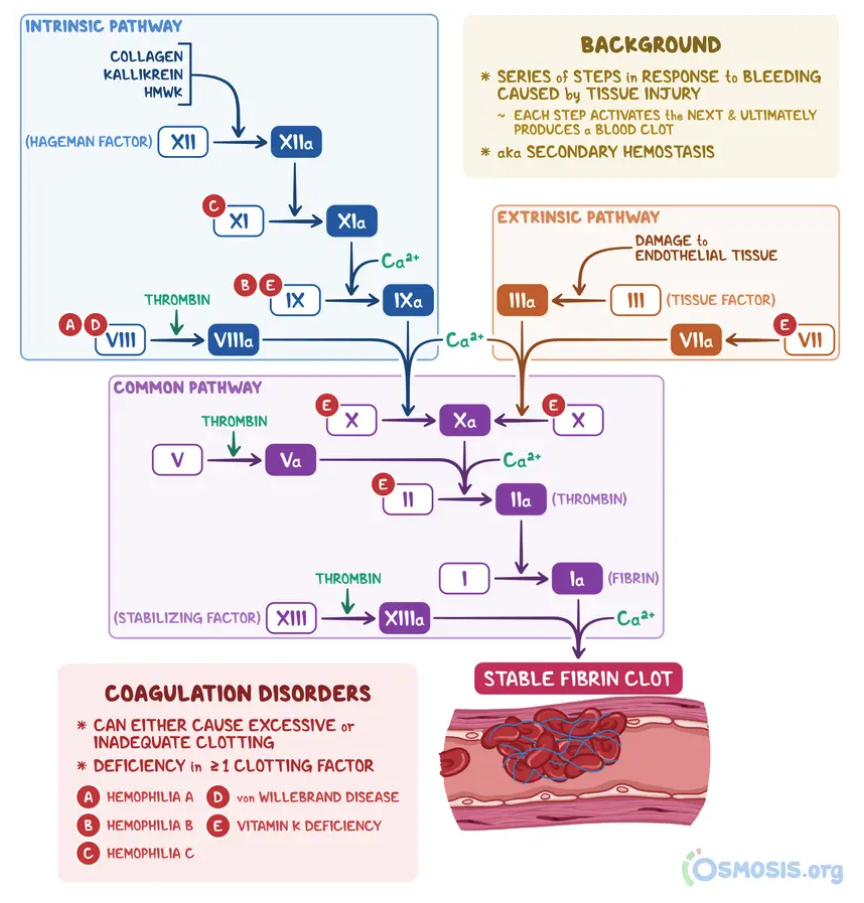
Coagulation Cascade:
NONE
What is the mechanism of action of unfractionated heparin?
Activates antithrombin (ATIII)—> which inhibits thrombin (IIa) and factor Xa—> decreasing clot formation.
What lab test is used to monitor unfractionated heparin therapy?
aPTT (activated partial thromboplastin time)
How is unfractionated heparin administered?
IV (intravenous)
What is the half-life of unfractionated heparin?
Short half-life
What are the main indications for unfractionated heparin?
Acute DVT or PE
Acute coronary syndrome (ACS)
Pregnancy (does not cross placenta)
Perioperative anticoagulation
Bridging therapy when initiating Warfarin
Why is heparin safe to use in pregnancy?
It does not cross the placenta.
What are the adverse effects of unfractionated heparin?
Bleeding
Heparin-induced thrombocytopenia (HIT)
Osteoporosis (with long-term use)
Hyperkalemia (due to aldosterone suppression)
What is heparin-induced thrombocytopenia (HIT)?
An immune-mediated reaction where 🩸 heparin binds to platelet factor 4 (PF4) → the body forms IgG antibodies against this complex.
These antibodies cause ⚡️ platelet activation, leading to:
🧱 Thrombosis (clot formation)
📉 Low platelet count (thrombocytopenia)
⚠ Increased risk of dangerous clots (DVT, PE, stroke)
🧠 Key concept: Even though platelets drop, the blood becomes hypercoagulable → meaning more clots, not more bleeding.
What are the contraindications for unfractionated heparin?
Active bleeding
History of HIT
Severe thrombocytopenia (platelets < 50,000)
High bleeding-risk procedures (e.g., neurosurgery)
What agent is administered to reverse Heparin’s anticoagulant effects in cases of significant bleeding?
Protamine Sulfate (*almost identical to heparin)
Whats the MOA of Protamine Sulfate?
💊 Protamine Sulfate is a positively charged molecule that binds to the negatively charged Heparin, forming a stable inactive complex that neutralizes Heparin’s anticoagulant effect.
By inactivating Heparin, Protamine restores normal activity of clotting factors that Heparin normally inhibits — specifically Factor IIa (Thrombin) and Factor Xa ⚖
Summary:
🧩 Protamine (+) + Heparin (−) → Inactive complex → Allows normal clotting via Factors IIa & Xa.
How much Protamine Sulfate do you need to reverse 100 units of Heparin?
💉 1 mg Protamine = reverses 100 units of Heparin
What is the maximum dose of Protamine Sulfate that can be given at one time?
🚫 Do not exceed 50 mg at any one time (risk of hypotension or anaphylaxis).
What are Low-Molecular Weight Heparin (LMWH drugs) medications?
*LMWH: smaller
Enoxaparin (Lovenox) *most common
Dalteparin
Tinzaparin
Nadroparin
LMWH activates Antithrombin III (ATIII) → inhibits Factor IIa (Thrombin) and Factor Xa ⚖
How does LMWH compare to unfractionated heparin in terms of half-life?
LMWH has a longer half-life ⏱
1⃣ Less Frequent Dosing
Because LMWH stays active longer in the body, it only needs to be given once or twice daily, instead of every few hours like unfractionated heparin (UFH).
➡ Improves convenience + patient compliance 🕐Allows less frequent dosing, predictable anticoagulation, lower HIT risk, and outpatient use ⏱💉🏠
What is the route of administration for LMWH?
Administered subcutaneously (SC) 💉 (pt administers)
What lab values should be monitored with LMWH therapy?
aPTT or anti-Factor Xa activity (for dose monitoring in special cases) 🧪
What is the primary mechanism target for LMWH visualized in the diagram?
LMWH binds to Antithrombin (ATIII) and inactivates Factor Xa and Factor IIa (Thrombin) → ↓ coagulation cascade
What are the main indications for LMWH?
Bridging therapy when transitioning to Warfarin ⚖
DVT/PE (treatment or prophylaxis) 🦵🫁
Acute Coronary Syndrome (ACS) or Myocardial Infarction (MI) ❤
What are the adverse effects of LMWH?
Thrombocytopenia (HIT)
Hyperkalemia (due to aldosterone suppression) ⚡
What are the contraindications for LMWH use?
Active bleeding 🚫
Severe thrombocytopenia (Platelet count < 50,000)
High bleeding risk procedures, e.g., neurosurgery 🧠🔪
💊 CM – UFH vs LMWH Flashcards 🧬🩸
Q: What’s the main difference between UFH and LMWH?
UFH: Large molecule → acts on Factor IIa (Thrombin) and Xa equally
LMWH: Smaller fragments → primarily inhibits Factor Xa > IIa
🧩 Mnemonic: “LMWH = Light on Thrombin”
How do UFH and LMWH differ in administration and onset?
Property | UFH | LMWH |
|---|---|---|
Route | IV or SC | SC only |
Onset | Instant (IV) ⚡ | ~30 min delay ⏳ |
Use setting | Hospital | Outpatient friendly 🏠 |
🧠 LMWH = “Less Monitoring, More Home use”
Which has a longer half-life and why is that beneficial?
LMWH has a longer half-life → once or twice-daily dosing ⏱
✅ Improves compliance
✅ Stable anticoagulation
✅ Allows outpatient therapy
Which requires more lab monitoring?
UFH: Requires aPTT monitoring 📉
LMWH: Usually no routine monitoring (anti-Xa level only in special cases — pregnancy, obesity, renal failure)
Why is LMWH’s effect more predictable?
Less protein binding → more predictable anticoagulant response
Greater bioavailability 💉
✨ Mnemonic: LMWH = “Little Molecule, More Predictable”
Which heparin type is more easily reversed?
UFH → fully neutralized by Protamine Sulfate ⚖
LMWH → partially reversed only 🚫
🧠 Mnemonic: “Unfractionated = Undo Fast”
Compare adverse effects between UFH and LMWH.
Effect | UFH | LMWH |
|---|---|---|
HIT (Heparin-Induced Thrombocytopenia) | Higher risk ⚠ | Lower risk ✅ |
Osteoporosis (long term) | Higher risk 🦴 | Lower risk ✅ |
Hyperkalemia | Both possible ⚡ |
When should LMWH be avoided?
Active bleeding 🚫
Severe thrombocytopenia (Plt < 50k)
High-risk bleeding surgeries (e.g. neurosurgery) 🧠🔪
Severe renal impairment (due to prolonged half-life) 💧
What are the main indications for LMWH?
Bridging to Warfarin ⚖
DVT/PE treatment or prophylaxis 🦵🫁
ACS / MI management ❤
🔟 Summary Memory Trick
🧠 “UFH = Unpredictable, IV, Immediate, In-Hospital”
🧠 “LMWH = Longer, Less Monitoring, More Home-friendly”
CM – Warfarin
What is the role of Vitamin K in coagulation?
Required for activation of clotting factors II, VII, IX, X and proteins C & S 🩸
Activation occurs via gamma-glutamyl carboxylase using reduced Vitamin K
Vitamin K Epoxide Reductase (VKORC1) converts Vitamin K from oxidized → reduced form ♻
What is Warfarin’s mechanism of action?
Inhibits VKORC1 (Vitamin K Epoxide Reductase) 🚫
↓ synthesis of active clotting factors II, VII, IX, X
Also inhibits Proteins C & S → causes initial hypercoagulable state ⚠
🧠 Mnemonic: “Warfarin stops the Vitamin K recycling factory → no active clotting factors!”
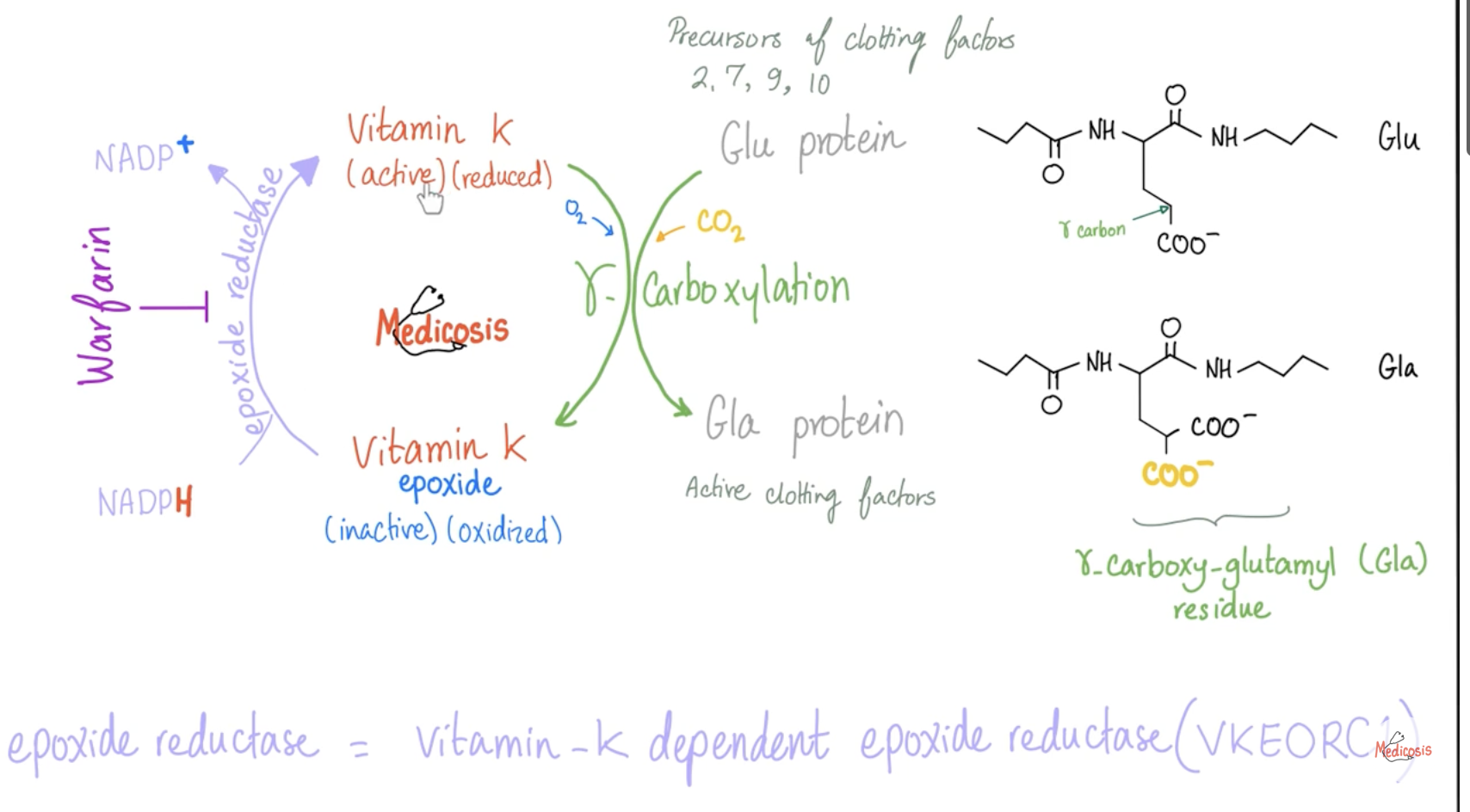
What enzyme does Warfarin inhibit?
Vitamin K Epoxide Reductase (VKORC1) 🚫—> prevents vitamin K to reduced form—> all factors in inactive form so you can’t use factors for clot formation. (fibrinogen—> fibrin to form clot)
Why does Warfarin cause an initial hypercoagulable state?
Proteins C & S (natural anticoagulants) are inhibited first → temporary imbalance → ↑ clot risk early in therapy
➡ Bridge with Heparin during initiation 🧩
What are the indications for Warfarin use?
DVT/PE prevention and treatment 🦵🫁
Stroke prevention in atrial fibrillation or mechanical/prosthetic valves ❤
Hypercoagulable states (e.g., Protein C/S deficiency, Antiphospholipid syndrome) ⚠
Which clotting factors are Vitamin K–dependent and affected by Warfarin?
Factors II, VII, IX, X + Proteins C & S
What lab test is used to monitor Warfarin?
PT/INR (Prothrombin Time / International Normalized Ratio) 📊
🧠 Goal INR: 2–3 (most cases)
What is the antidote for Warfarin toxicity?
Vitamin K (phytonadione) 💉
Fresh Frozen Plasma (FFP) or Prothrombin Complex Concentrate (PCC) for severe bleeding 🩸
What type of state can occur if Warfarin is given without bridging?
Transient hypercoagulable state → may cause skin necrosis due to Protein C depletion.
What are the adverse effects of Warfarin?
☠ Bleeding – most common major complication
Skin necrosis – due to early Protein C depletion → transient hypercoagulable state
Purple toe syndrome – caused by cholesterol embolization 🦶💜
💡 Explanation: Warfarin inhibits both clotting factors and natural anticoagulants (Proteins C & S), leading to early clot formation in skin vessels before full anticoagulation takes effect.
What is the main contraindication for Warfarin?
🚫 Pregnancy (teratogenic) — may cause fetal bleeding and bone defects
❗Exception: May be used if the patient has a mechanical heart valve ❤
💡 Explanation: Warfarin crosses the placenta and interferes with fetal Vitamin K–dependent proteins, but benefit may outweigh risk in high-thrombosis conditions.
Skin Necrosis from Warfarin
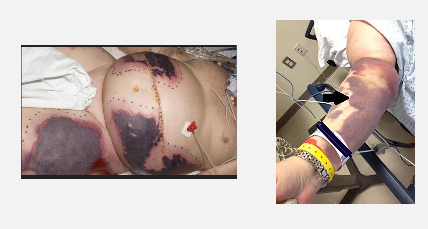
A 72-year-old male on Warfarin presents with gum bleeding and bruising.
INR = 8.5 (supratherapeutic), normal Hb, platelets, and creatinine.
What are the next steps in management?
➡ Hold Warfarin temporarily
➡ Administer oral Vitamin K (phytonadione)
➡ Monitor INR and reassess within 24–48 hrs
💡 Explanation: INR 8.5 with mild bleeding = over-anticoagulation but not severe → reversing with oral Vitamin K is safest.
How do you manage supratherapeutic INR depending on the level and bleeding status?
INR Level | Bleeding? | Management |
|---|---|---|
4.5–10 | ❌ No | Hold Warfarin only |
>10 | ❌ No | Hold Warfarin + Oral Vitamin K |
Any INR | ✅ Yes | Stop Warfarin + IV Vitamin K + PCC (Prothrombin Complex Concentrate) 💉 |
CM – Warfarin Bridge Flashcards 💊🩸Why do patients starting Warfarin require a bridge with a second anticoagulant (e.g., Heparin or LMWH)?
Because Warfarin takes 5–7 days to reach its full anticoagulant effect ⏱
➡ During this time, Protein C & S levels drop first → causing a transient hypercoagulable state ⚠
➡ A bridge prevents clot formation while waiting for therapeutic INR levels.
What is the purpose of bridging therapy when starting Warfarin?
Prevents thrombotic events during the initial hypercoagulable period 🩸
Bridge (usually Heparin or LMWH) is continued until INR is therapeutic (2–3) for at least 24 hours ✅
💡 Mnemonic: “Bridge the gap until Warfarin works!”
What are the key steps in Warfarin bridging therapy?
1⃣ Start Warfarin and continue for at least 5 days.
2⃣ Begin Heparin or LMWH on Day 1 💉
→ You don’t give Warfarin alone because it can make the patient temporarily hypercoagulable, increasing the risk of skin necrosis.
→ This occurs since Warfarin inhibits Protein C and S (natural anticoagulants) before it reduces synthesis of Factors II, VII, IX, and X.
3⃣ Continue both (Heparin + Warfarin) until INR ≥ 2.0 for at least 24 hours.
4⃣ Once INR is stable → Stop Heparin/LMWH.
💡 Purpose: Maintain full anticoagulation while Warfarin takes effect and prevent early clot formation.
Why must Heparin or LMWH be used when starting Warfarin?
Because Warfarin lowers Protein C & S first (within ~7 hours), before it fully inhibits clotting factors II, VII, IX, X (which take 48+ hours).
➡ This creates a short hypercoagulable window, risking thrombosis or skin necrosis ⚠
➡ Heparin “bridges” this gap by providing immediate anticoagulation.
🧠 Mnemonic: “Heparin Holds the Line While Warfarin Warms Up!”
How does the timeline of clotting factor depletion explain the need for bridging?
A:
Protein C & S: Fast decline (~7 hours) → early clot risk
Factors II, VII, IX, X: Slow decline (24–60 hours) → full anticoagulation delayed
➡ Heparin covers this lag until Warfarin reaches steady therapeutic INR
When can you safely stop Heparin bridging?
When INR ≥ 2.0 for at least 24 hours while on combined therapy ✅
💡 Goal: Ensure Warfarin’s anticoagulation is stable before removing Heparin support.
What drugs are Direct Oral Thrombin Inhibitors?
Bivalirudin, Argatroban, Dabigatran (💡“BAD” mnemonic)
What is the mechanism of action of Direct Oral Thrombin Inhibitors?
A:
→ Directly inhibit thrombin (IIa)
→ Faster than warfarin
→ No INR monitoring required
They directly inhibit thrombin (factor IIa), preventing the conversion of fibrinogen to fibrin.
How do Direct Oral Thrombin Inhibitors compare to warfarin in terms of onset and monitoring?
They act faster than warfarin and do not require INR monitoring.
What are the main clinical indications for Direct Oral Thrombin Inhibitors?
→ DVT/PE treatment & prophylaxis
→ Stroke prevention (non-valvular atrial fibrillation)
→ Can be used in HIT (when heparin is BAD)
What are the major adverse effects associated with Direct Oral Thrombin Inhibitors?
Bleeding (reversible with idarucizumab)
GI complications: esophagitis, gastritis, GERD, GI ulcers
What antidote reverses the effects of Direct Oral Thrombin Inhibitors like Dabigatran?
Idarucizumab — a specific reversal agent for Dabigatran. 💉
What are the contraindications to using Direct Oral Thrombin Inhibitors?
Renal impairment (CrCl < 30 mL/min)
Bleeding diathesis
💊 DOACs – Apixaban, Edoxaban, Rivaroxaban
What does DOAC stand for, and what are examples of these medications?
A: DOAC = Direct Oral Anticoagulant → includes Apixaban, Edoxaban, Rivaroxaban.
What is the mechanism of action of DOACs?
They directly inhibit Factor Xa, reducing thrombin generation.
Do DOACs require anticoagulation monitoring?
No, anticoagulation monitoring (like INR) is not required.
What is the onset of action for DOACs?
Rapid onset: 1–4 hours.
What are the main clinical indications for DOACs?
DVT/PE treatment and prophylaxis
Stroke prophylaxis (non-valvular atrial fibrillation)
Post-orthopedic surgery thromboprophylaxis
What are the major adverse effects of DOACs?
Bleeding (reversible with PCC or andexanet alfa)
GI bleeding (higher risk with rivaroxaban)
What are the contraindications for DOACs?
Active bleeding
Pregnancy or breastfeeding
Mechanical heart valve
Antiphospholipid syndrome
Hepatic impairment
Severe renal impairment (CrCl < 25 mL/min → avoid)
🩸 Anticoagulant Reversal Agents
What is the reversal agent for Warfarin?
Vitamin K
Mechanism: Restores production of vitamin K–dependent clotting factors (II, VII, IX, X).
What are two additional agents that can reverse Warfarin toxicity?
Prothrombin complex concentrate (PCC or K-Centra) – provides clotting factors II, VII, IX, X.
Fresh Frozen Plasma (FFP) – replenishes clotting factors.
What is the reversal agent for Unfractionated Heparin (UFH) and Low Molecular Weight Heparin (LMWH)?
A: Protamine Sulfate
Mechanism: Binds heparin to neutralize its anticoagulant effect.
What is the reversal agent for Direct Thrombin Inhibitors (e.g., Dabigatran)?
A: Idarucizumab (Praxbind)
Mechanism: Monoclonal antibody that binds and inactivates dabigatran.
What is the reversal agent for Factor Xa Inhibitors (Apixaban, Edoxaban, Rivaroxaban)?
A: Andexanet alfa (Andexxa)
Mechanism: Binds and inactivates Factor Xa inhibitors.
🧠 Anticoagulant Reversal Summary Chart
Anticoagulant | Reversal Agent | Mechanism of Action | Notes |
|---|---|---|---|
Warfarin | Vitamin K | Restores production of Vitamin K–dependent clotting factors (II, VII, IX, X) | Slower onset |
Warfarin | PCC (K-Centra) | Provides clotting factors II, VII, IX, X | Rapid reversal |
Warfarin | Fresh Frozen Plasma (FFP) | Replenishes clotting factors | Used if PCC unavailable |
UFH / LMWH | Protamine Sulfate | Binds heparin to neutralize its effect | Partial reversal for LMWH |
Direct Thrombin Inhibitor (Dabigatran) | Idarucizumab (Praxbind) | Monoclonal antibody binds & inactivates dabigatran | Specific & fast |
Factor Xa Inhibitors (Apixaban, Edoxaban, Rivaroxaban) | Andexanet alfa (Andexxa) | Binds & inactivates Factor Xa inhibitors | Short-acting; IV use only |
🩸 Case 1 – Pulmonary Embolism (PE)
Questions:
What imaging finding suggests a pulmonary embolism?
In a hemodynamically stable patient with PE, what is the first-line treatment?
When should an IVC filter be used in PE?
What is the management for hemodynamically unstable PE?
After treatment begins, the patient’s hemoglobin drops from 12.5 → 8.8 g/dL with hypotension and melena. What complication is suspected?
What are the next steps in management for anticoagulant-induced bleeding?
CT angiography shows a filling defect within the pulmonary arteries, consistent with PE.
IV Unfractionated Heparin (UFH) or LMWH.
When anticoagulation is contraindicated (e.g., active bleeding).
Thrombolytic therapy (Alteplase, tPA, Streptokinase, Urokinase). If contraindicated → embolectomy (surgical or catheter-directed).
Upper GI bleeding due to anticoagulant therapy.
→ Stop anticoagulant.
→ Reverse agent: Protamine sulfate for heparin; Andexanet alfa for Factor Xa inhibitors.
→ Provide blood transfusion and hemodynamic support.
→ Perform urgent EGD (endoscopy) to identify and control bleeding source.
🧠 Case 2 – Heparin-Induced Thrombocytopenia (HIT)
Questions:
What clinical finding raises suspicion for Heparin-Induced Thrombocytopenia (HIT)?
What is the 4T score used for?
What should be done if the 4T score is intermediate or high?
What diagnostic test confirms HIT?
What should be done immediately when HIT is suspected?
Which medication should be started after discontinuing heparin?
Answers:
Decreased platelet count after recent heparin exposure.
To estimate the probability of HIT (Heparin-Induced Thrombocytopenia).
Discontinue LMWH or heparin immediately and evaluate further.
Heparin-induced antibody test (e.g., PF4-heparin ELISA or serotonin release assay).
Stop all heparin products (including flushes and LMWH).
Start a direct thrombin inhibitor such as Argatroban.