S5 - The Cytoskeleton and Cell Motility
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
general features of cytoskeleton
cytoskeleton elements are not membrane- bound
all cytoskeleton elements are polymers
microtubules = polymers of tubulin
micorfilaments = polymers of actin
intermediate filaments = variable
non-covalent linkages i.e. dynamic elements
cytoskeleton functions
structural support
framework to position organelles
movement of materials
cell motility
mitosis and cytokinesis
microtubules
largest fibres (25nm)
hollow tubes
stiff, hollow, inextensible tube that can resist bending when a cell is compressed
alpha-tubulin & beta-tubulin → heterodimers (noncovalent)
heterodimers → protofilaments
13 profilaments → microtubule
image of microtubules
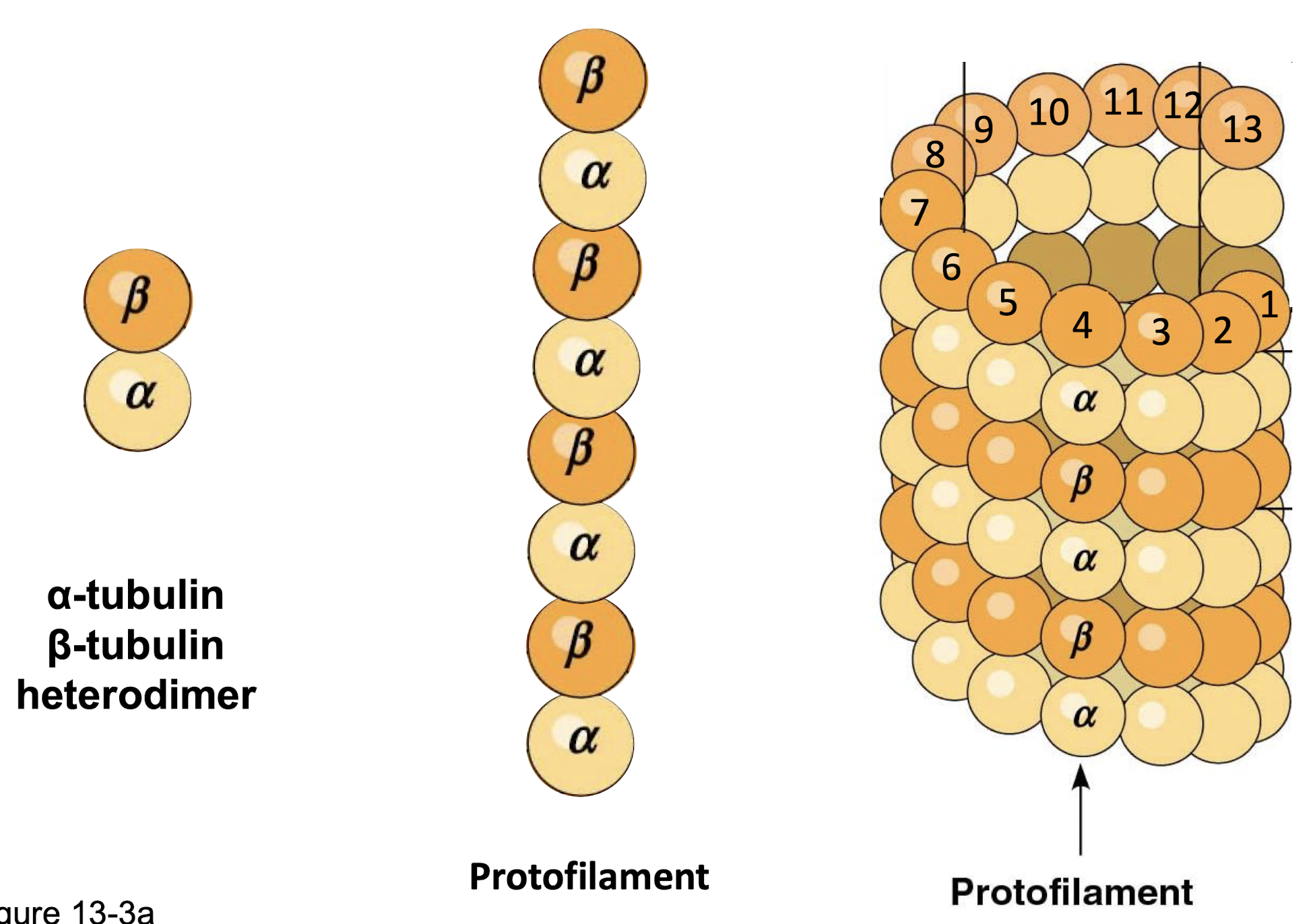
other structural feature of microtubules
polar filaments
plus end (bet subunit)
hydrolyze GTP → GDP
minus end (alpha subunit)
only GTP (not exchangeable)
Structural: Microtubule Associated Proteins (MAPs) function and types
increase stability of microtubules and promote their assembly
MAP1, MAP2, MAP4, tau
two types of dynampic MAPs
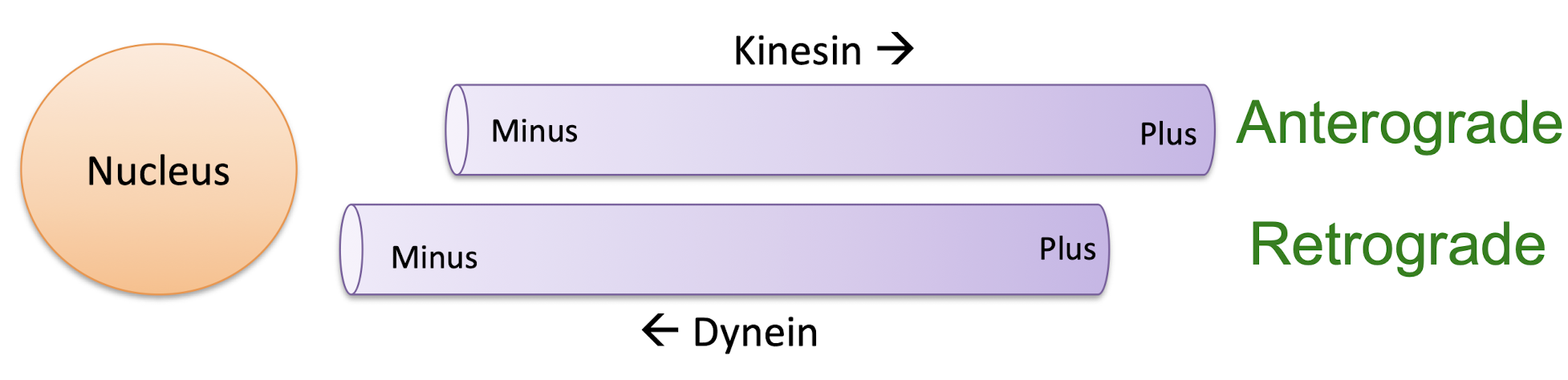
kinesin
move twds outside of cell
“+” end directed movement (kind/+)
dynein
moves twds inside of cell (dying/-)
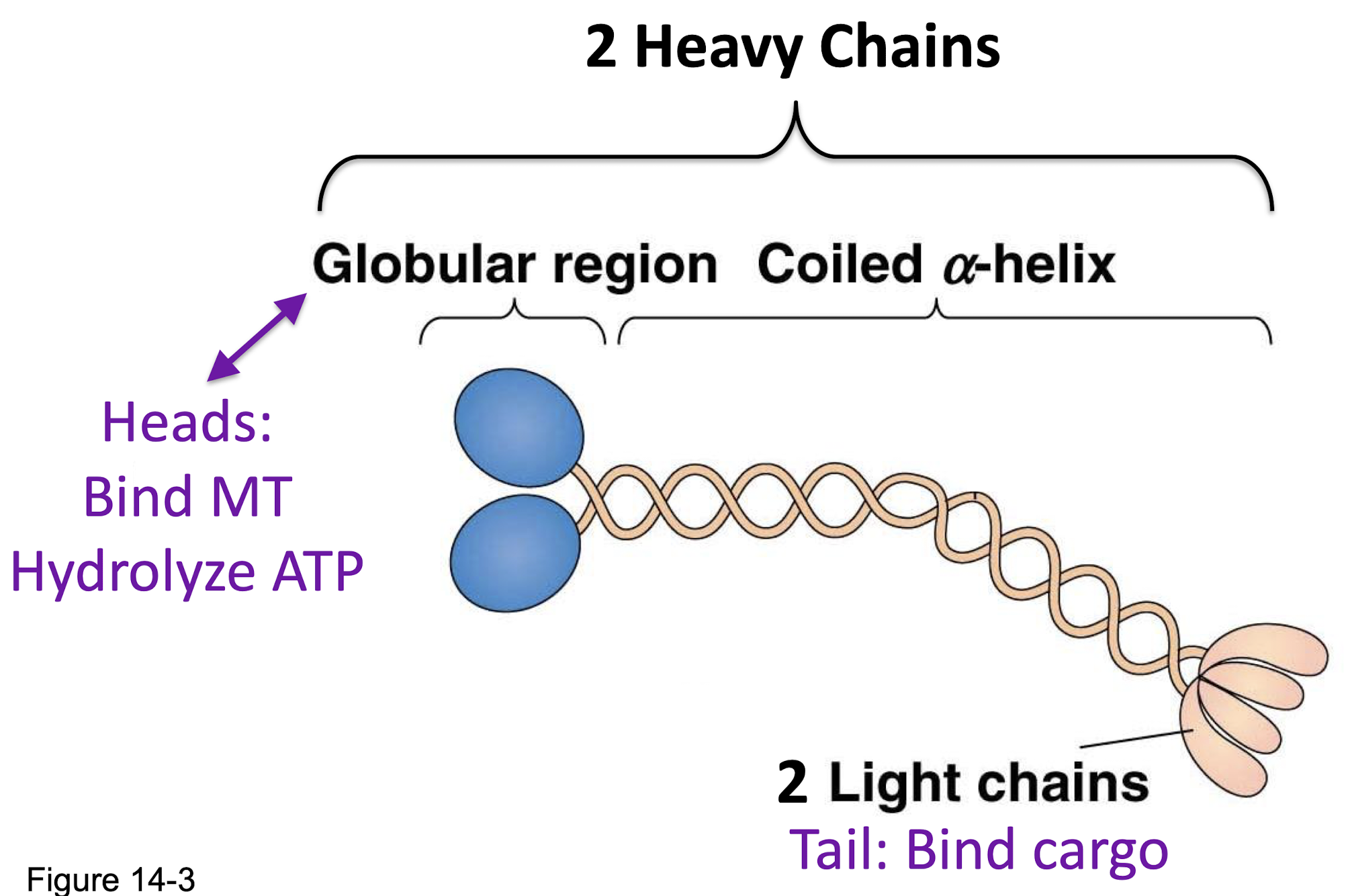
kinesin related proteins
~45 different kinesins
divided between 14 families
walk twds plus end (anterograde)
kinesin acceptions
kinesin-14 moves towards minus end
kinesin-13 doesnt move at all
kinesin-1
how does kinesin move?
“hand-over-hand” mechanism
“ATP Powers MT binding, ATP & ADP
cytoplasmic dynein
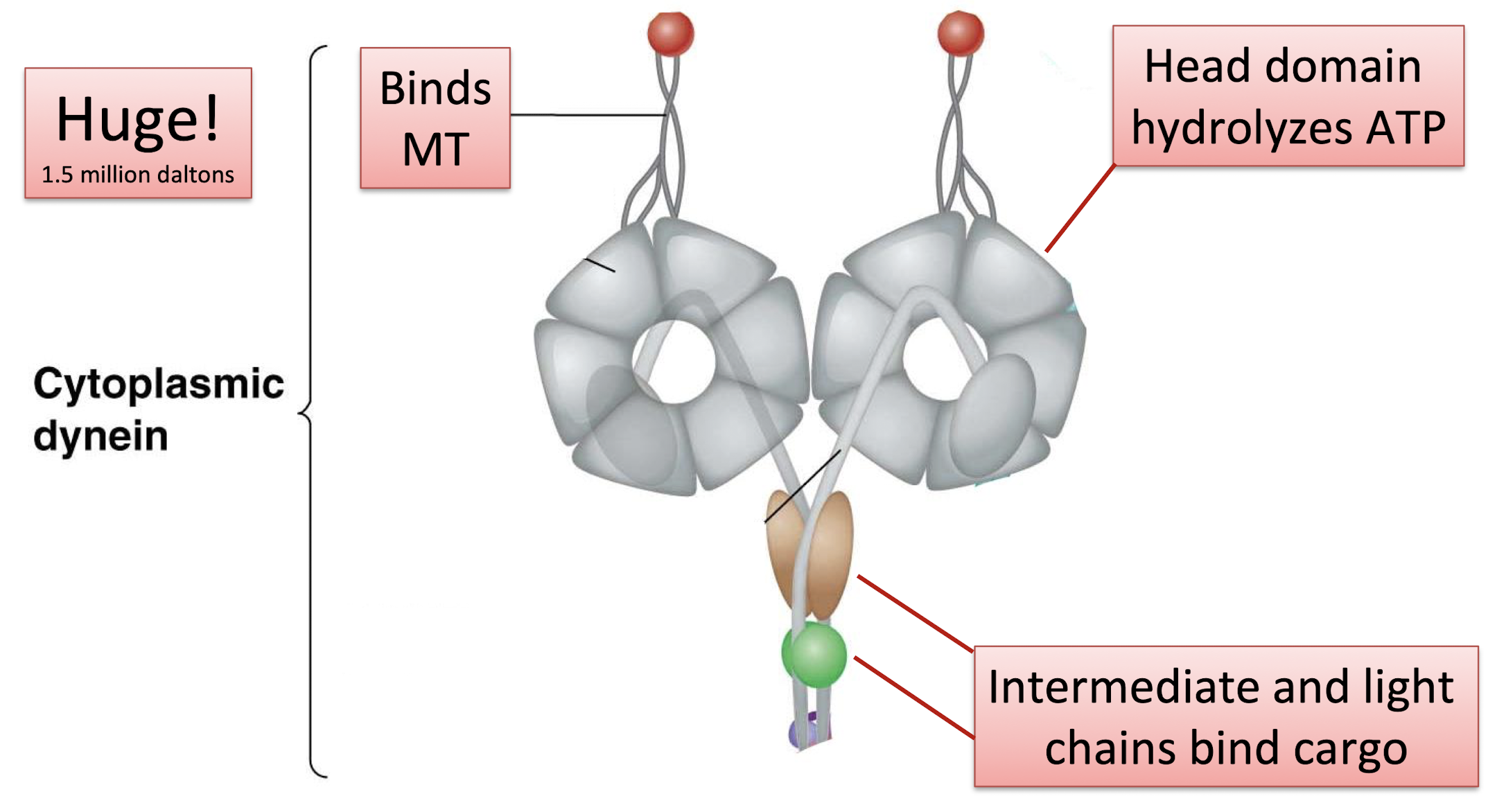
dynactin
required to bind cargo
function of microtubule-organizing centers (MTOCs)
to organize MT-associated structures and organelles
to orient kinesin-mediated and dynein-mediated transport of organelles, vesicles, and vesicular tubular clusters
location of microtubule-organizing centers (MTOCs)
often perinuclear (surrounding nucleus)
center of cell
nucleation
the initiation of growth of MTs
example of a MTOC
centrosome
centrosomes
two perpendicular centrioles (each made of nine triplet microtubules) + perientriolar material (PCM)
what is periecentriolar material (PCM)?
a diffuse granular matrix surrounding the centrioles
enriched with Gamma (beta) tubulin
what is PCM critical for?
microtubule nucleation
flagella: axoneme
“9+2” array
9 doublet microtubules
2 normal single microtubules
what does bending in cilia and flagella depend on?
crosslinks in the axoneme
basal body
MTOC for the axoneme
structurally identical to centrosome
intermediate filaments (IFs)
~10-12 nm
rope-like, not hollow
unique to animal cells
tough flexible, extensible, elastic filament
no role in motility
very stable, provides mechanical support
can withstand tensile forces
chemically heterogenous (at least 70 types, divided between 6 classes)
how is intermediate filament assemly different than that of microtubules and microfillaments?
new tetramers incorporated into middle throughout the length of the filament, not ends
neither ATP or GTP involved
instead relies on phosphorylation
phosphatase - remove PO4 cause assembly
kinases - add PO4 cause disasembly
nuclear lamina
thin, dense meshwork of fibers that lines the inner surface of the inner surface of the inner nuclear membrane nuclear membrane and helps support the nuclear envelope
plectin
an intermediate filament associated protein
will form bridges with IFs, MTs, MFs
keratins
tethered to the nuclear envelope and the outer edge of the cell
desmosomes and hemidesmosomes
role of keratin IFs in cell attachments
desmosomes
cell:cell attachment structures
hemidesmosomes
cell:ECM attachment structures
desmosomes
found in tissues subjected to mechanical stress, such as cardiac muscle, epithelial layers of the skin and uterine cervix
the network of intermediate filaments provides tensile strength to the entire sheet of cells
hemidesmosomes
keratin filaments extending outward into the cytoplasm
keratin mutations can lead to cell adherence disorders
microfilaments
smallest fibres (6-8nm)
made of actin protein
flexible, inextensible helical filament
as a contractile element a microfilament can generate tension
important for movement within cell and of the cell itself
microfilament functions
cell shape (cortex)
cell migration
transport of vesicles and organelles (esp in plants)
cytokinesis
muscle contraction
Actin structure
G-actin (globular actin) = monomer
lobes - each lobe has two domains, ATP bind in the cleft
F-actin (filamentous actin)= polymer
in mature filament, two F-actins wrap around each other to form a helical structure
binding of actin molecules, what is least and most stable?
binding provides polarity to the molecule
two = weak
three = stable
myosin
molecular motor of actin
plus end directed motors (i.e. towards the barbed end)
head domain → hydrolyses ATP
conventional (type II) myosins vs unconventional myosins
myosin II (conventional)
first ones discovered
2 heads and long tail
no cargo, they twist with eachother
form bipolar filaments
Myosin I or Myosin V (unconventional)
smaller
myosin I → single head
myosin V →two heads
no filament formation
tail binds vesicles and membrane
thick filament of skeletal muscle
hundreds of myosin II molecules
they twist with eachother
form bipolar filaments
muscle contraction
relaxed = myosin heads not interacting with actin microfilaments
contracted = myosin heads have pulled the actin closer
sacromere
muscle fiber
contains actin (thin) and myosin (thick)
how does myosin move?
ATP dependent process
like kinesin and dynein, use chemical energy to do work
two types of actin organization
both found in cell cortex
bundles - parallel fibers (often found in filopodia)
networks - can be 2D or 3D
what do actin-binding proteins do?
regulate polymerization and length of filaments
nucleating proteins
forms a nucleating center by mimicking the shape of actin subunits so G-actins will start to add
ex Arp2/3
monomer sequesting
controls amount of G-actin available for polymerization
ex thymosin beta4
end blocking (capping)
fillament grows or shrinks
prevent G-actin addition and loss
CapZ caps “+” end (filament will shirk)
Tropomodulin caps ‘minus’ end ‘minus’ end (filament will grow)
monomer polymerizing
increases actin filament growth rates
promotes G-actin addition to filament plus ends
ex profilin
depolymerizing proteins
binds the ‘minus’ (pointed) end and causes depolymerization
ex cofilin
cross-linking and bundling proteins
holds filament together
ex. filamin - holds filaments at right angles
ex. villin - holds filaments in parallel
filament severing protein
breaks up MF network causing the actin gel to soften and liquefy
caps newly-exposed plus ends to prevent further polymerization
ex. gelsolin
membrane binding
secures microfilament to the membrane so that the membrane follows actin movement
ex. dystrophin, vinculin