How are chemicals separated and tested for purity?
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
chemical analysis is
the instruments and methods we use to separate, identify, and quantity different substances
Formulation
a mixture that has been prepared using a specific formula for a particular purpose with a precise amount of diff components
Mixture
A combination of two or more elements that are not chemically combined
impure substances boiling and melting points
range of temps
A pure substance
A substance made of only one kind of matter and having definite properties.
pure substances melting and boiling points
specific sharp melting and boiling points
Paper chromatography
method of separating substances in a mixture of different colours.
Chromatography method
1. Draw a dot on base line drawn on chromatography paper
2. Fill up a beaker with water or ethanol
3. put chromatography paper in water without line touching solvent
4. Wait for ink to run and leave chromatogram to dry
Stationary phase of paper chromatography
The paper because molecules do not move
mobile phase of paper chromatography
The solvent used i.e water as molecules can move
Rf value formula
distance moved by substance/distance moved by solvent
a substance which is more soluble in mobile phase will..
spend more time in mobile phase
move faster and travel further distance up paper
pure substance in paper chromatography
would not separate but just moves up as single spot
solvent
a liquid in which substances can dissolve
Filtration
Separates insoluble solids from liquids using filter paper
Crystallisation
A separation technique used to produce solid crystals from a solution by evaporating the solvent.
Crystallisation method
1. A solution is placed in an evaporating basin and heated with a Bunsen burner.
2. The volume of the solution has decreased because some of the water has evaporated. Solid particles begin to form in the basin.
3. All the water has evaporated, leaving solid crystals behind.
4)cool and dry crystals
Simple distillation
Used to separate a liquid from a solution
i.e pure water from sea water
Distillation method
A solution is heated, usually using a Bunsen burner.
The liquid in the mixture evaporates into a gas.
The gas is cooled by a water jacket, and condenses into a liquid, which then flows into the beaker.
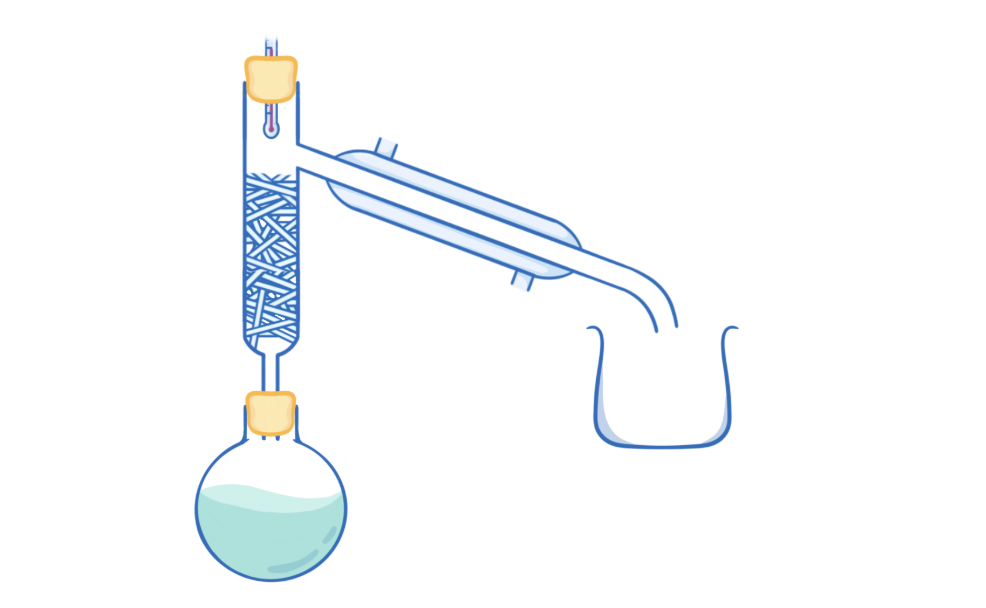
Fractional distillation
Used to separate a pure liquid from a mixture of liquids
Fractional distillation method to separate multiple liquids
-heat is applied and liquid with lowest bp evaporates and condenses into beaker
-if other liquids evaporate by chance, they condense in the fractionating column back into flask
-temp is altered to repeat process for second liquid
-three liquids are separated bases on diff BP with third remaining in flask