RP10 - Aspirin
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
What is recrystallation?
used to purify impure solids
a hot solvent is used to dissolve the organic solid and its impurities, the solution will cool which crystallises the solid and leaves the impurities in the solution
using a minimum amounts of solid to dissolve the solid and avoid loss of product
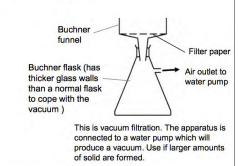
a Buchner funnel can be used to speed up this process using reduced pressure
after filtration the product is washed with a cold solvent and dried on filter paper
What does the melting point of a solid indicate?
indicates its purity and identity
can be matched to known substances
impurities lower the melting point and broaden the melting point range
How is aspirin prepared?
6g salicylic acid added to a conical flask with 10cm³ of ethanoic anhydride with 5 drops of concentrated sulfuric acid
mixture is swirled and kept in a warm water bath for 20 minutes
the flask is then allowed to cool and the contents are added to a cold water beaker which allows the aspirin to crystallise
aspirin recovered using a Buchner funnel and left to dry
Why is reflux used?
it allows an organic reaction mixture to be heated without losing reactants or products
Why are anti bumping granules used?
prevents vigorous and uneven boiling by making small bubbles
Why is a minimum volume of hot solvent used?
dissolve impure compound in a minimum volume of hot solvent, minimum volume used to obtain a saturated solution and to allow cooling for the desired compound to be pure in crystals
What is the method for recrystallisation?
dissolve impure compounds in minimum volume of hot solvent
hot filter through a filter paper to remove insoluble impurities
cool the filtered solution using a beaker in ice to crystalise desired product and keep soluble impurities in the solution
use a Buchner funnel to filter the solution using reduced pressure to separate crystals
wash crystals with distilled water and allow to dry
Why may there be a loss of yield?
crystals lost during filtering or washing
some product stays in the solution after recrystallisation
side reaction
What is the set up for the Buchner funnel?