8.8 redox reaction
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
what is a redox reaction
when electrons are transferred between substances at the same time
displacement reactions and redox
displacement reactions are a type of redox,
metal ion always gains electrons and is reduced
metal atoms always lose electrons and get oxidised
displacement reactions and redox
zinc + copper sulfate =zinc sulfate +copper
Zn (s) +CuSo(2)(s) =ZnSo(4)(aq) +Cu(s)
zinc loses 2 electrons to become 2+ ion -is oxidised zinc is more reactive then copper,electrons (2e-) stay at metal surface
copper ions gain those electrons-Cu2+ ions in solution take electrons that zinc released,the copper ions are reduced(they gain electrons) and become solid copper metal forming redish layer on zinc strip
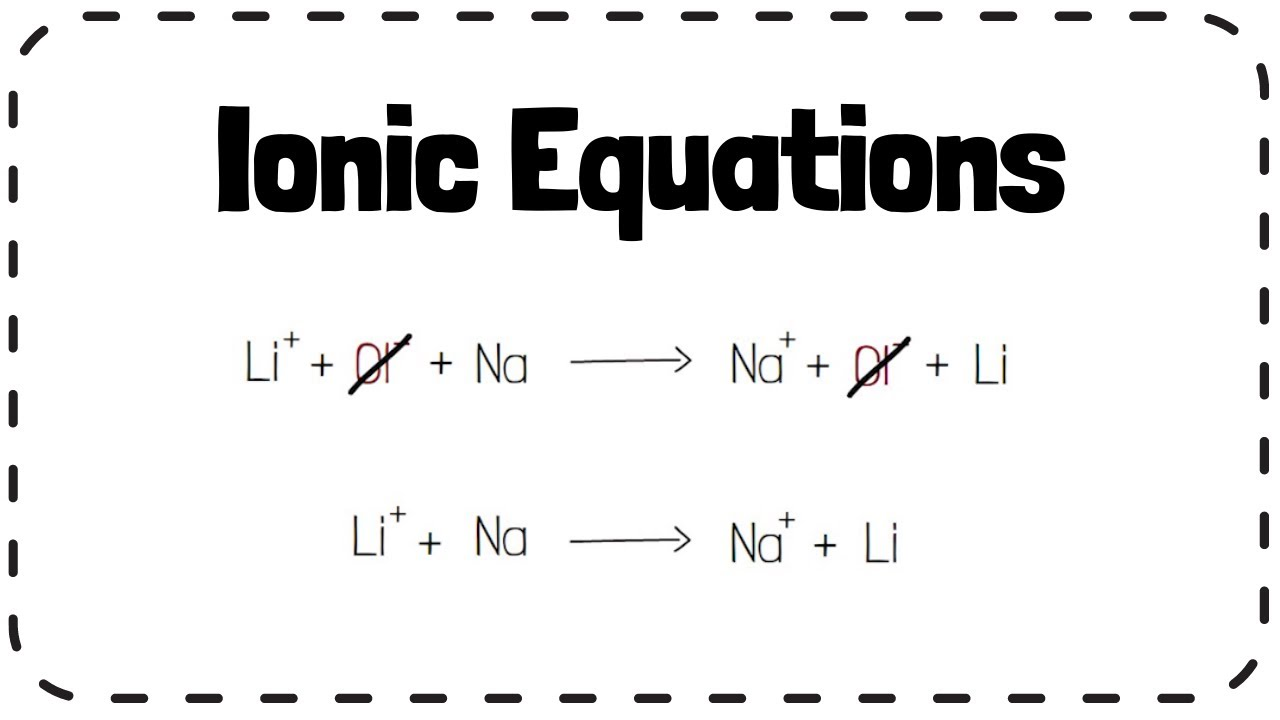
what do ionic equations show
only the particles that react and the particles they form
what do ionic equations for redox concentrate on
substances which are oxidised or reduced
ionic equations of displacement reactions
chloride ions dont change in the reaction and so can be ignord
Fe(s) + 2HCI(aq) =FeCL(2)(aq) + H(2)(g)
iron and hydrochloric acid react to form iron chloride and hydrogen
Fe(s) + 2H(+)(aq) =Fe(2+)(aq) +H(2)(g)
iron atoms oxidised to lose two electrons and to Fe(2+) and H(+) ions reduced to hydrogen