Data Analysis revision
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
Tests for Carbon double bonds
Bromine test
Baeyer’s test
Iodine test
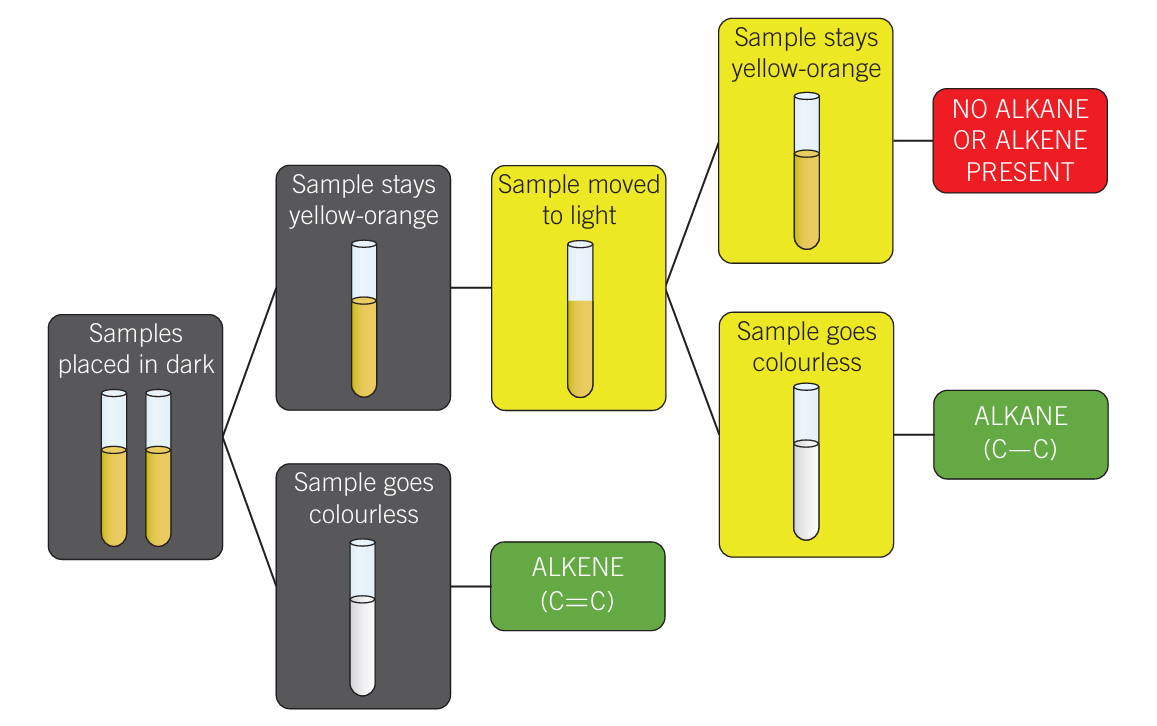
Bromine Test
Qualitative test
Bromine is typically dissolved in water and added to the unknown sample in the dark
Alkanes react to UV light
Colourless in dark = Alkene
Stays yellow/orange in dark then colourless in UV light = Alkane
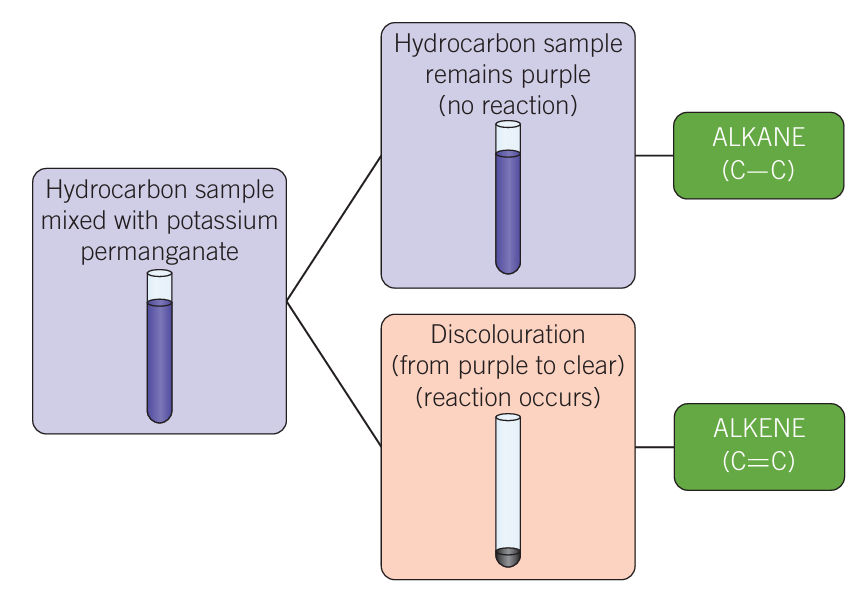
Baeyer’s test
qualitative test
Hydrocarbon sample mixed with potassium permanganate (purple sample)
Goes colourless = alkene
Stays purple = alkane
Iodine test
Quantitative test
determine number of Carbon - Carbon double bonds
Calculate mols of each reactant then divide each by lower mol value = number of carbon-carbon double bonds
Tests for hydroxyl groups
oxidation of alcohols
Esterification of alcohols
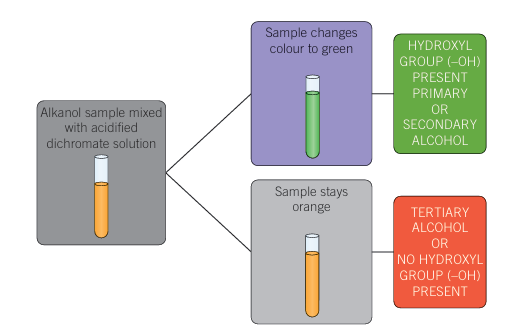
Oxidation of alcohols
Only primary or secondary alcohols can be oxidised
Oxidising agent (acidified dichromate) is added – colour change noted
Stays orange = tertiary alcohol OR no hydroxyl group
Colour changes to green – hydroxyl group present, primary OR secondary
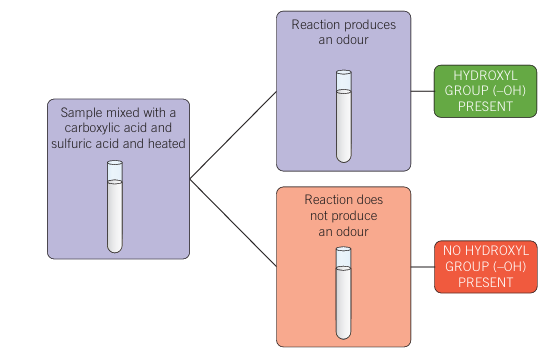
Esterification of alcohols
- alcohol and acid react to form an ester
- carboxylic acid added to unknown compound In presence of sulfuric acid at high temps
- Odour produced = hydroxyl group
- No odour = no hydroxyl group
Tests for Carboxyl groups
- Carboxylic acids contain both hydroxyl and carboxyl group
- Esterification
- Testing for acidity
- Reaction with metal carbonate
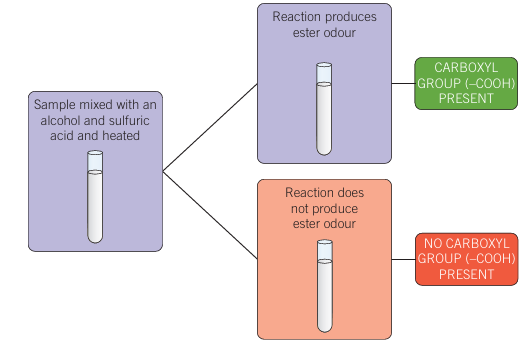
Esterification of carboxylic acids
- Alcohol added to the unknown compound in presence of sulfuric acid at high temps
- sweet odour = carboxyl group
- No odour = no carboxyl group
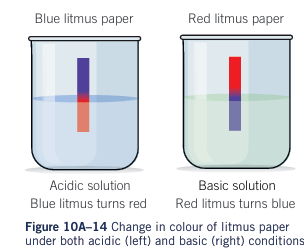
Testing for acidity
- Carboxylic acids are weak acids (Ph below 7)
- Blue litmus will turn red
Reaction with metal carbonate
- Acid reacts with metal carbonate to form metal salt, CO2 and H2O
- Generation of bubbles
- CO2 bubbled through limewater will turn cloudy
Volumetric analysis terms
Titrant: solution of a known concentration
Analyte: solution of unknown concentration
Standard solution: solution of precisely known concentration
Performing a titration
Reducing agent with the unknown concentration is added to the conical flask using a pipette
An indicator is added to solution
The oxidising agent, with known concentration is added to the burette. The initial volume reading is recorded
The tap of the burette is turned to flow
Once the indicator changes colour, the tap is stopped. The final volume on the burette is recorded. The total volume added to the conical flask is recorded. Process repeated until 3 concordant titres are achieved
The total volume of the solution required for the redox reaction to go to completion is used to calculate the unknown concentration
oxidising/reducing agent for titration
Reducing agent = unknown concentration, placed in conical flask
Oxidising agent = known concentration, placed in burette
Burette rinsed with water
Titrant becomes diluted, more titrant is required.
Concentration of:
Conical flask: Overestimated
Burette: Underestimated
Pipette rinsed with water
Analyte in conical flask becomes diluted
Concentration of:
Conical flask: Underestimated
Burette: Overestimated
Conical flask rinsed with solution to fill it
Solution in conical flask has a higher concentration of solute
Concentration of:
Conical flask: Overestimated
Burette: Underestimated
Missed indicator point
Bigger discrepancy between end point and equivalence point
Concentration of:
Conical flask: Overestimated
Burette: Underestimated
Preparing equipment for titration
Rinse the:
the volumetric flask with deionized water
the pipette and burette with the solution they will hold.
What is Mass spectrometry
- Powerful analytical technique involving ionisation fragmentation, separation and detection
- Gives information about the masses and abundances of atoms and molecules in a sample
Ionisation and fragmentation
- When the sample is injected into the mass spectrometer, it is bombarded with high energy electrons, which knock off one or more electrons and form positive ions
- These ions are unstable, and can fragment further, breaking one or more bonds in the [M]+ ion
FRAGMENTS MUST BE SHOWN AS IONS
![<p><span>-</span><span style="font-size: 7pt; font-family: "Times New Roman";"> </span>When the sample is injected into the mass spectrometer, it is bombarded with high energy electrons, which knock off one or more electrons and form positive ions<br>- These ions are unstable, and can fragment further, breaking one or more bonds in the [M]<sup>+</sup> ion</p><p>FRAGMENTS MUST BE SHOWN AS IONS</p>](https://knowt-user-attachments.s3.amazonaws.com/2251e18a-d9dc-48db-ba31-1e8da6a2cf84.png)
Separation and detection
- Once fragmentation has occurred, the fragments are accelerated through the spectrometer
- Separation occurs as objects of different masses will have different acceleration
- The fragments hit the detector, which plots the mass to charge (M/Z) ratio against the percentage abundance of that fragment
- Only charged species are detected
- The m/z value is obtained by dividing the mass of the fragment by its charge (typically +1)
Mass spectrometry terminology
Base peak: The most common fragment (tallest) – assigned a percentage abundance of 100%
Molecular (parent) ion peak: The ions that corresponds to the molecular ion (end peak). This m/z ratio helps determine the molar mass
Ratios for isotopes
Chlorine: 3:1 (35,37) – gap of 2 m/z
Bromine: 1:1 (79,81) – gap of 2 m/z
Infrared spectroscopy
- Powerful analytic technique that involves the interaction of various covalent bonds with infrared radiation
- Gives information about the types of bonds in a molecule – functional groups that may be present
- When molecules absorb infrared radiation, it causes the atoms and bonds to vibrate
- The frequency the bonds are vibrating at is directly proportional to the energy that bond absorbs
- Frequency is measured in cm-1 and is called a wavenumber
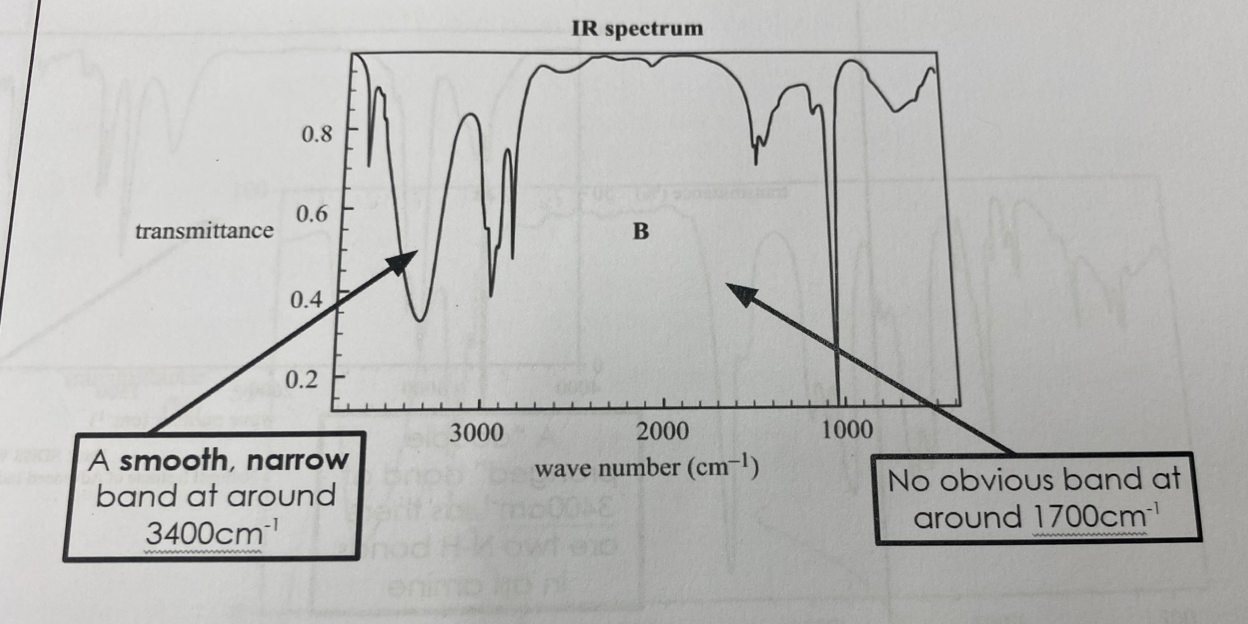
Alcohols
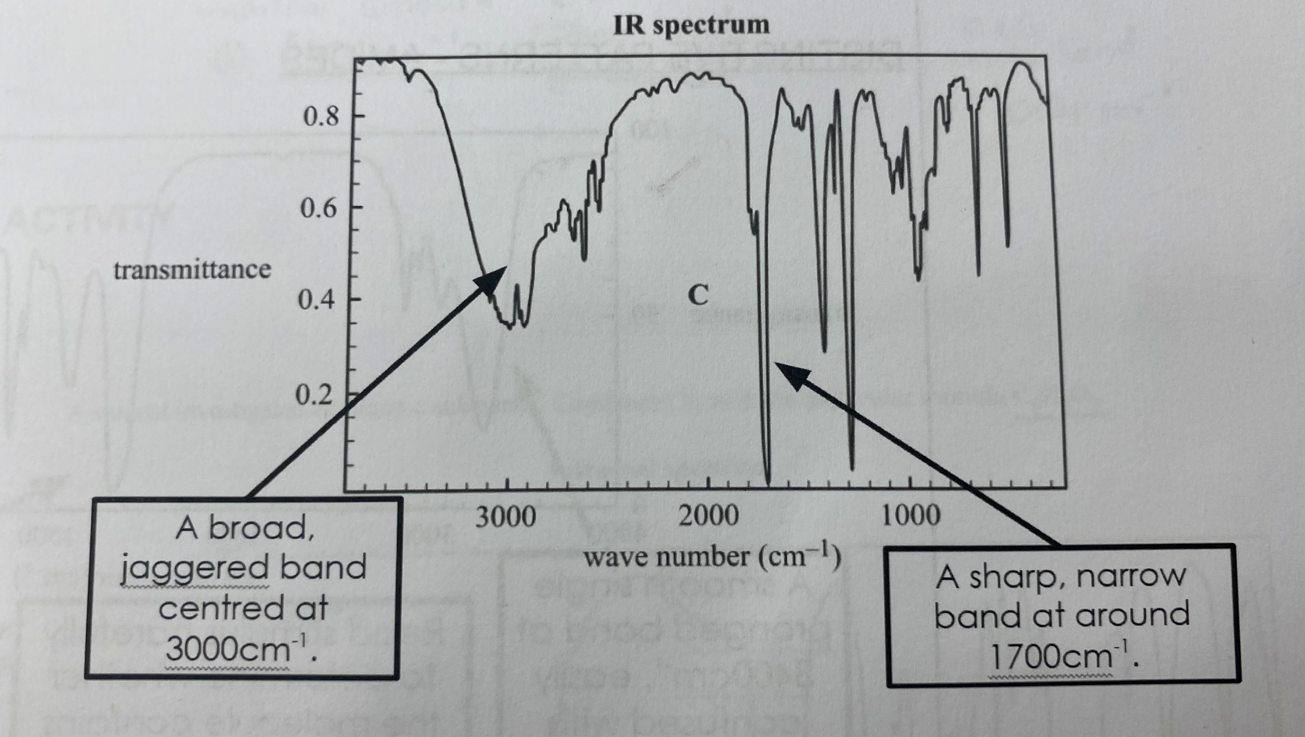
Carboxylic acids
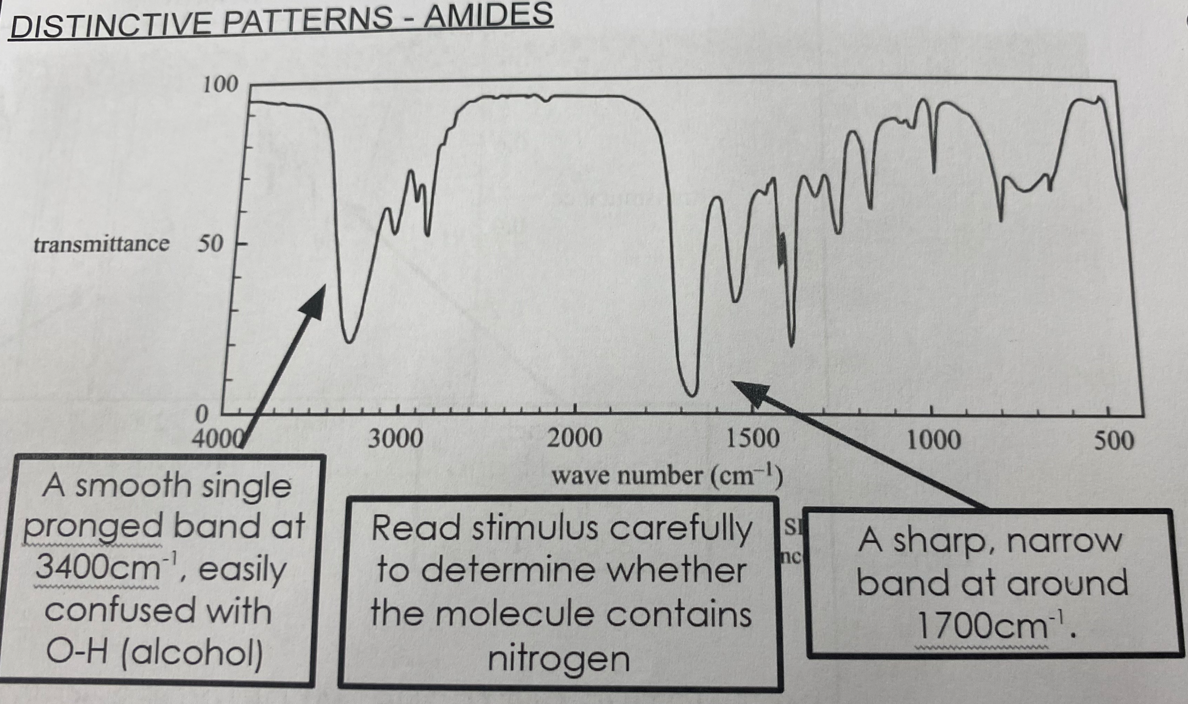
Amides
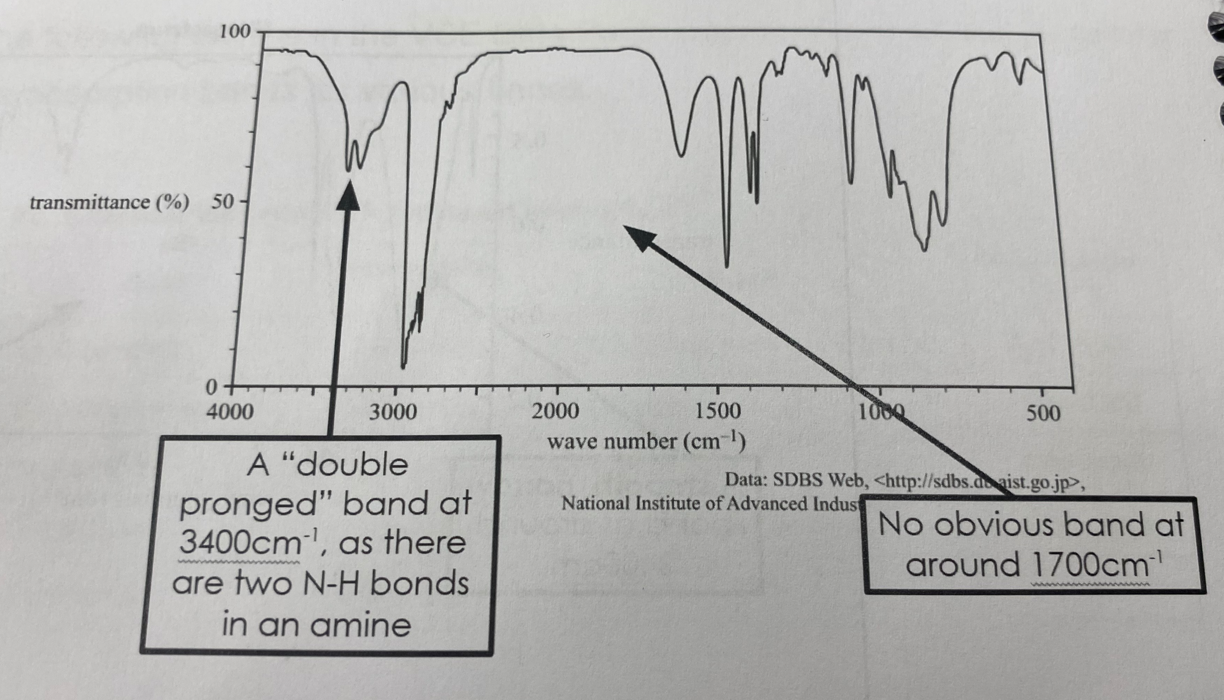
Amines
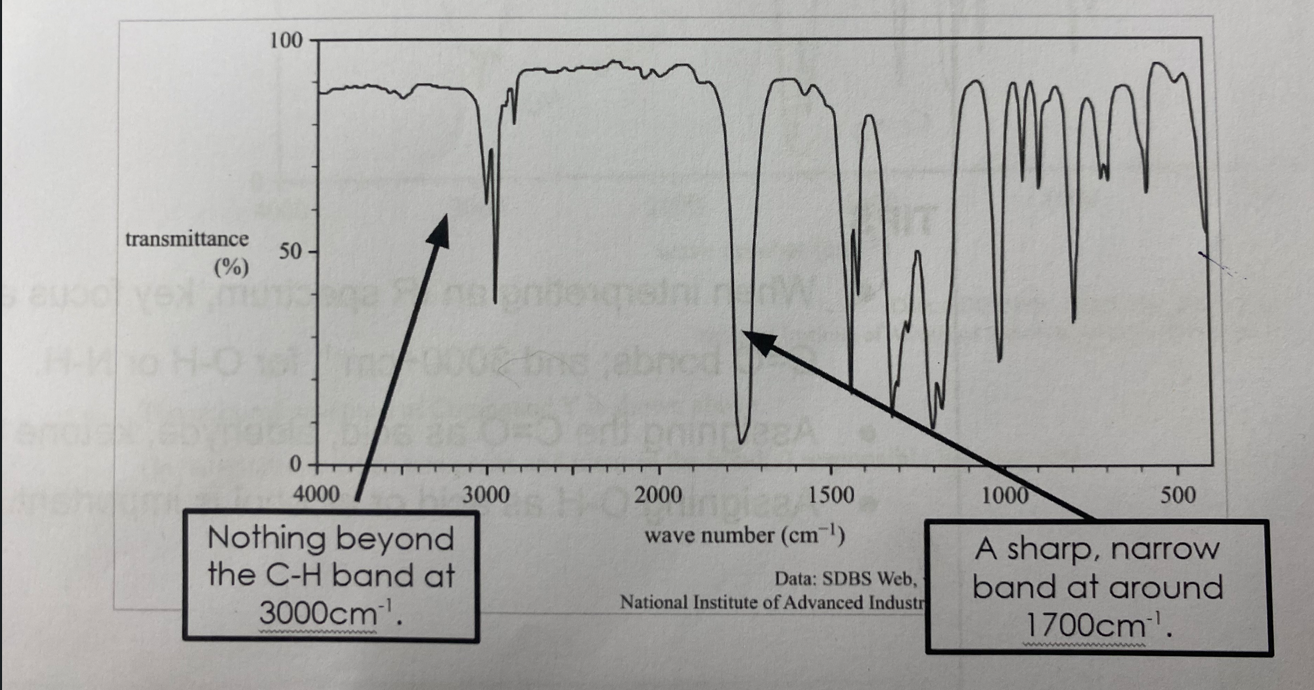
Aldehydes, Ketones and Esters
What is nuclear magnetic resonance (NMR)
When a magnetic field is applied to the nuclei, they can absorb and emit electromagnetic radiation at certain frequencies
This is because the spins of the nuclei can flip between the two energy levels, and the energy difference between these levels determines the frequency of electromagnetic radiation that can be absorbed or emitted
By measuring these frequencies, NMR can provide information about the structure and dynamics of molecules,
Chemical environments
every atom in the same chemical environment produces a single signal in an NMR spectrum
Atoms have the same environment if they are attached to the same atoms in the same way
Chemical shift and shielding
Chemical shift is the difference in energy needed to change the spin state in a sample compared to the energy required to change spin states in TMS
An electronegative atom decreases the nuclear shielding of the surrounding chemical environments; therefore, they have a higher chemical shift
Why is TMS a good reference
Inert
contains one hydrogen and one carbon environment
Highly shielded
splitting patterns
in high resolution HNMR, splitting patterns tell how many hydrogens are in the neighbouring hydrogen environment through n+1 rule
Hydrogens bonded to heteroatoms (not C-H) are always singlets
Integration curves
plot of the intensity of the NMR signal as a function of the chemical shift. The integration curve provides information about the relative number of hydrogen atoms that produce each signal in the NMR spectrum
the height of each environment
High performance liquid Chromatography
a powerful analytic technique that involves the separation of components within a mixture based on their polarity and attraction the stationary and mobile phases
Stationary phase
a solid, liquid or gel that remains static (in place)
In HPLC, stationary phase is typically polar - meaning polar components will stick and have a higher retention time
Mobile phase
The liquid or gas that flows and transports the compounds to be separated at different rates over the stationary phase
In HPLC, the mobile phase is non-polar - meaning non-polar components will stick and have a higher retention time
Retention time
The amount of time it takes for the compound to pass through the column
Absorption & Desorption
Absorption: The adhesion of molecules to the surface of a solid (stationary phase)
Desorption: The release of an absorbed substance (back into the mobile phase)
Explain how a HPLC machine can separate components based on their polarity
HPLC separates components based on their polarity.
The more polar components will adsorb readily to the highly polar stationary phase. This will cause the component to stay within the column for longer, increasing retention time.
The less polar components will desorb more readily into the less polar mobile phase. This will cause the component to move quickly through the column, decreasing retention time.
Purity
the degree to which a substance or compound is free from contaminants or other unwanted materials
Methods to assess purity
Melting point test
distillation
spectroscopy
Melting point test
the melting point of pure compounds will be very narrow - normally 1-2 degrees C
Impure samples will melt at a lower temperature and have a larger range, due to the disruption of intermolecular forces within the substance
Spectroscopy for purity
Mass spectroscopy: peaks that do not correlate to the molecular ions indicate impure sample
Infrared spectroscopy: absorption bands uncharacteristic of the desired molecule indicate impure sample (such as OH bands indicate presence of water)
NMR spectroscopy: peaks uncharacteristic, not well defined, indicate an impure sample
Distillation
The process of simple distillation is often used to separate and purify different chemicals based on their boiling points
Solvent extraction
based on separation by polarity
immiscible solvents are added, shaken. The solvent particles align with their polarities. solvents separated by decanting
then purified by distillation
Stereoisomerism
Molecules have the same structural formula, but the way the atoms are arranged in a 3d space is different
optical isomers
two molecules that are non-superimposable mirror images of each other
A molecule that CAN be superimposed on its mirror image is achiral
Identifying chiral centres
a carbon that has four unique groups attached to it
Note:
Any carbon that is bonded to two or more H is achiral
Any carbon involved in a double bond is achiral
Medical importance of chiral centres
arrangement of atoms in a 3d space is critical for biological interactions
Molecules must be able to interact in the correct orientation with correct charge attraction for maximal interactions
if molecules do not fit precisely into the active site of the enzyme, the biological process does not occur optimally
Enzymes
Biological catalysts that are not used up in the reaction and provide an alternate reaction pathway with a lower activation energy. They do not alter the position of the equilibrium and are classified as proteins as they are made up of many amino acids bonded together
Amino acids
building blocks of proteins
an amino acid is a 2-amino carboxylic acid containing both the acidic carboxylic group and the basic amino group
Amine attached to a carbon (which is connected to a H and a variable group) which is connected to the carboxyl group
Zwitterions
A ion with both a positive and negative charge simultaneously
amino acid exists as a zwitterion is neutral conditions
amino acids act as bases in low pH (accepts protons) - both functional groups are protonated
amino acids act as acids in high pH (Lose protons) - both functional groups are deprotonated
Forming polypeptides
condensation polymerisation
2 amino groups are condensed into a dipeptide and water. This process is repeated
Primary structure
the individual sequence of amino acids in the polypeptide backbone as joined by peptide links (amide bonds)
Secondary structure
Gives rise to two different orientations: alpha helix, beta pleated sheets
involve hydrogen bonding between peptide links
Tertiary structure
determines the overall 3d shape of the molecule
The tertiary structure refers to the other types of bonds the peptide can make using the side groups or R groups”
if the R groups arenon polar: dispersion forces
Polar: dipole-dipole interactions
Contains sulphur: disulphide bridge
Protonated/deprotonated: ionic bond
N-h, O-H or C=O: hydrogen bond
Quaternary structure
combinations of two or more different polypeptide chains
Contain the same interactions as tertiary
Lock and key model
only specific substrates can use the enzyme
Exothermic reactions break down large molecules with the release of energy
Endothermic reactions build larger molecules from smaller ones, requiring energy input
Changing temperature of enzymes
All enzymes typically have an optimal temperature at which they function (37 degrees)
- Temperature is below that optimal temperature, the enzyme activity decreases as the kinetic energy of the molecule decreases
- When the temperature is above the optimal temperature, the enzyme activity decreases due to denaturing
Denaturing
the permanent alteration of the secondary, tertiary and quaternary structures of a protein. Caused by the increasing temperature disrupting the intermolecular forces that hold together the protein
Changing pH
Enzymes have an optimal pH at which they function (7)
- any change to pH will denature an enzyme
Competitive inhibition
Enzymes catalyse a process by allowing the substrate to bind to its active sight
if the active sight is blocked by an inhibitor, the enzyme will not be able to function
useful in medicinal applications as inhibitors stop an enzyme from working (e.g. slowing down among of cholesterol produced in liver)