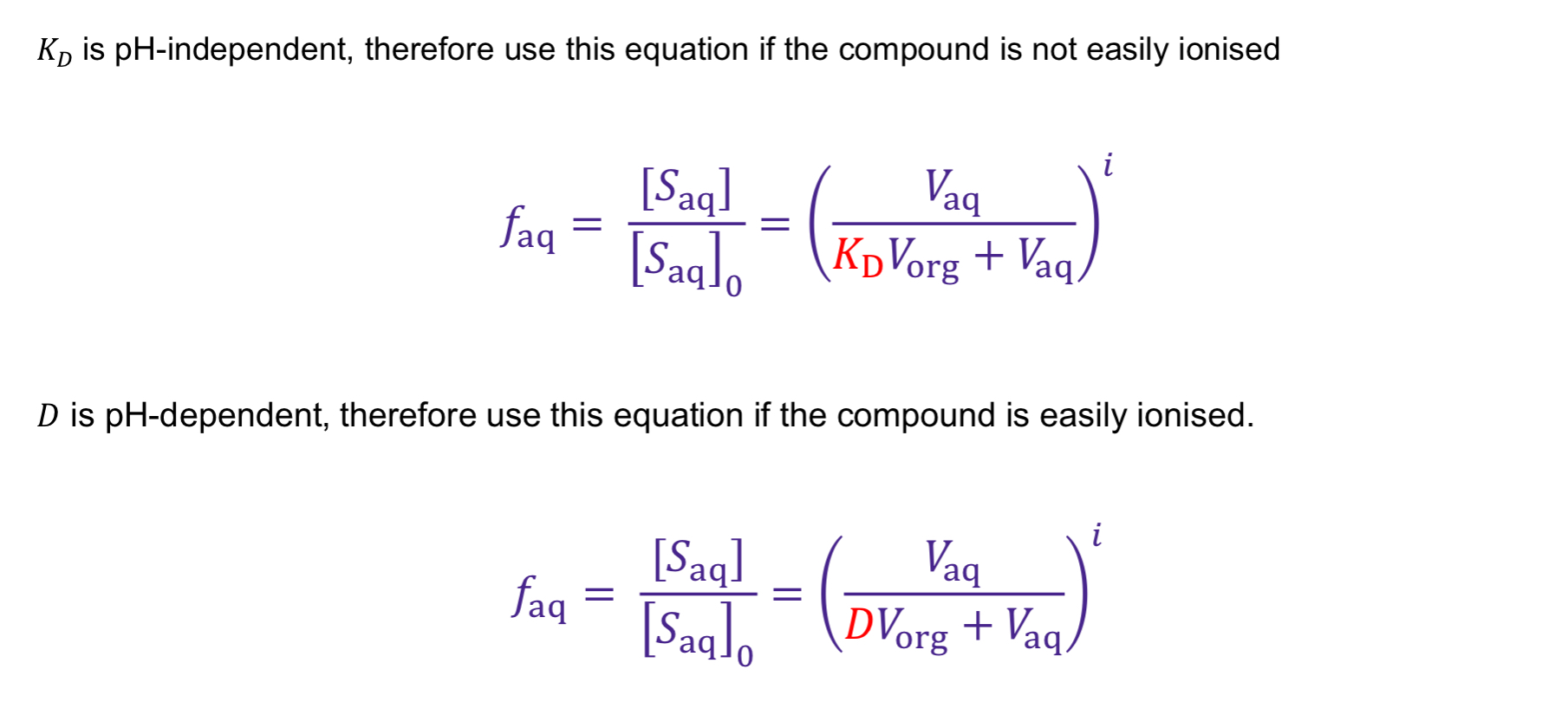Solvent Extraction
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is partitioning?
What is the equation for the partition coefficient/distribution coefficient?
[Sorg] = organic solvent conc
[Saq] = aqueous solvent conc
![<p>[Sorg] = organic solvent conc</p><p>[Saq] = aqueous solvent conc</p>](https://knowt-user-attachments.s3.amazonaws.com/ccf27a84-def6-4dd8-9c76-ba95f0c0f2c6.jpg)
What is the relationship between solubility and KD?
KD > 1
more soluble in organic
KD = 1
equally soluble in both
KD < 1
more soluble in aqueous
Why is it more efficient to do multiple extractions at smaller volumes?
Continuously halves the concentration of the aqueous solvent, more solute is removed
What is extraction efficiency?
f(aq) = fraction of aqueous solvent left behind
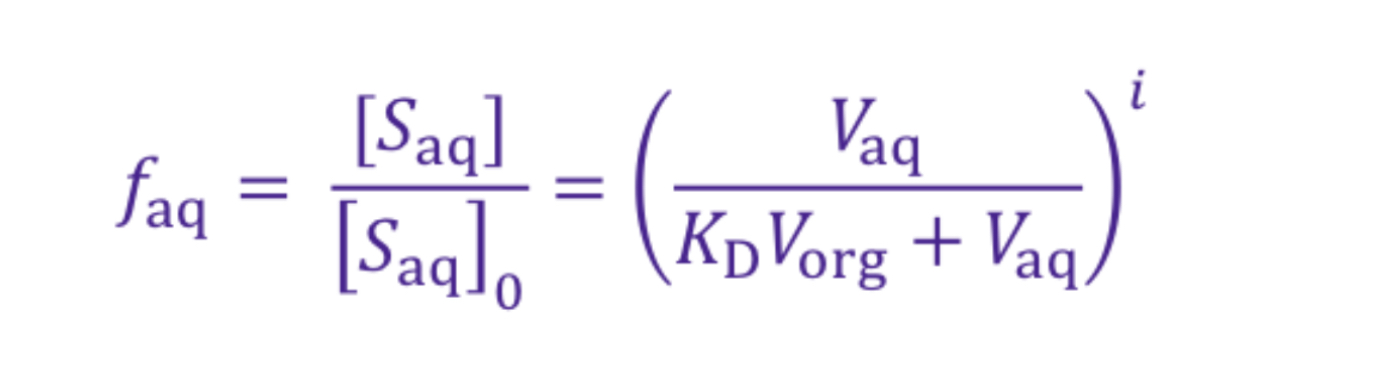
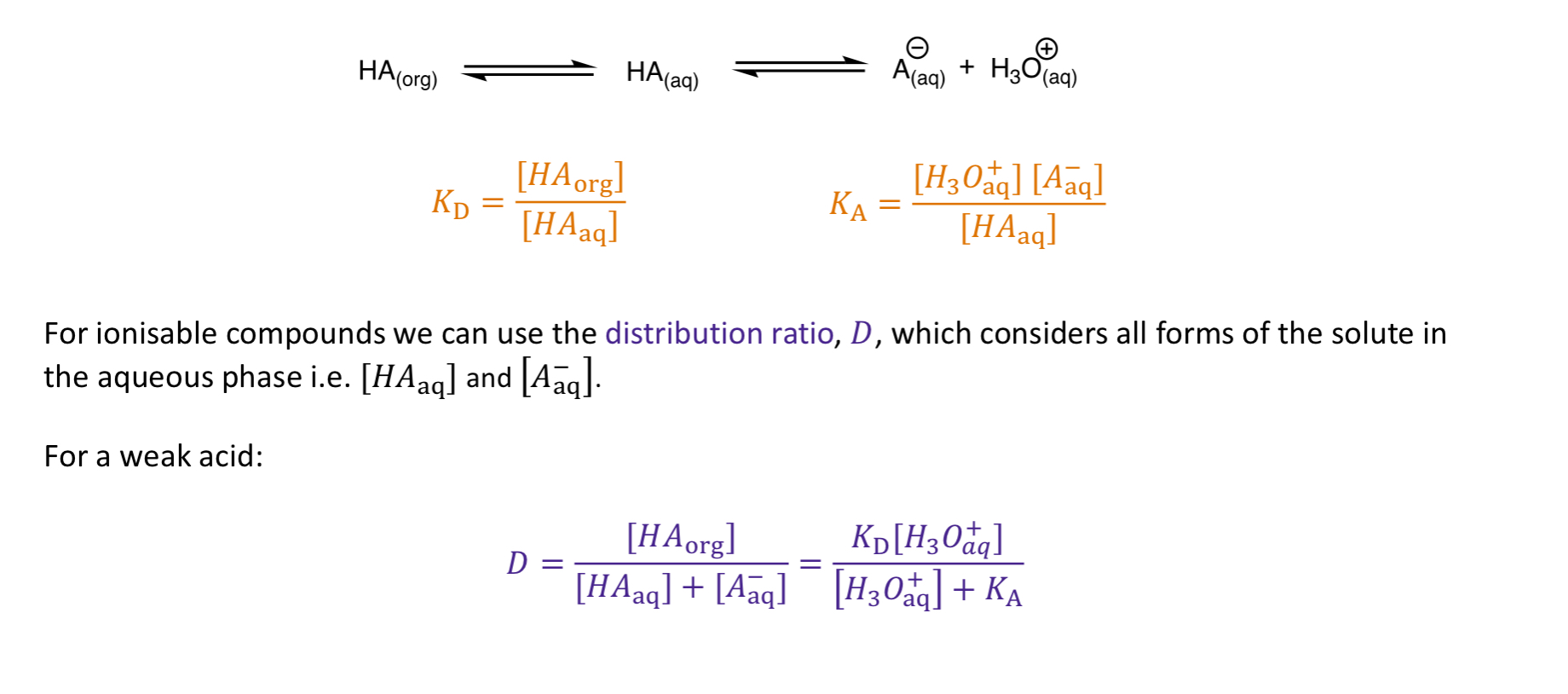
How would partitioning work for weak acids?
DISSOLVE IN ORG = protonate w/ acid (HCl)
DISSOLVE IN AQ = deprotonate w/ base (NaOH)
*NOTE: ionised species MORE soluble in aq than non-ionised
How is the distribution ratio calculated for weak acids?
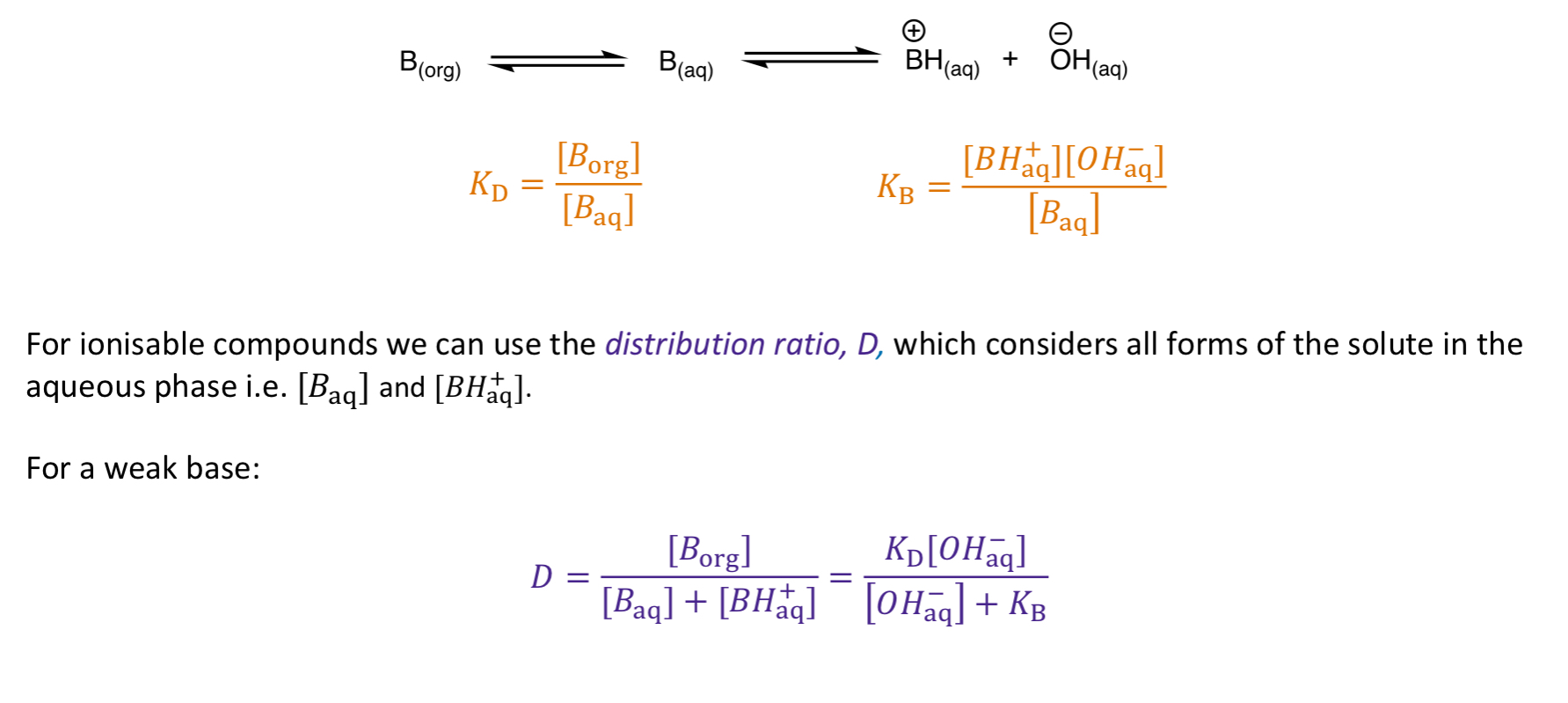
How would partitioning work for weak bases?
DISSOLVE IN ORG = deprotonate w/ base (NaOH)
DISSOLVE IN AQ = protonate w/ acid (HCl)
How is the distribution ratio calculated for weak bases?
What are the two different extraction efficiency equations (faq) used when dealing with easily ionisable and not easily ionisable substances?
KD = pH independent
compounds NOT easily ionsed
D = pH dependent
compounds EASILY ionised