GEAS (CHEMISTRY AND MATERIAL SCIENCE- PROBLEM SOLVING)
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms

Zinc (Zn) is a silvery metal that is used in making brass (with copper) and in plating iron to prevent corrosion. How many grams of Zn are in 0.356 mole of Zn? Given: molar mass of Zn = 65.39 g/mol
23.3 g Zn

Calculate the molecular mass (in amu) of caffeine (CsHIoN402). Given: C-12.01 amu; H=1.008 amu; N=14.01 amu; 0=16.00 amu A. 184.2 amu B. 194.2 amu C. 201.2 amu D. 240.2 amu
194.2 amu
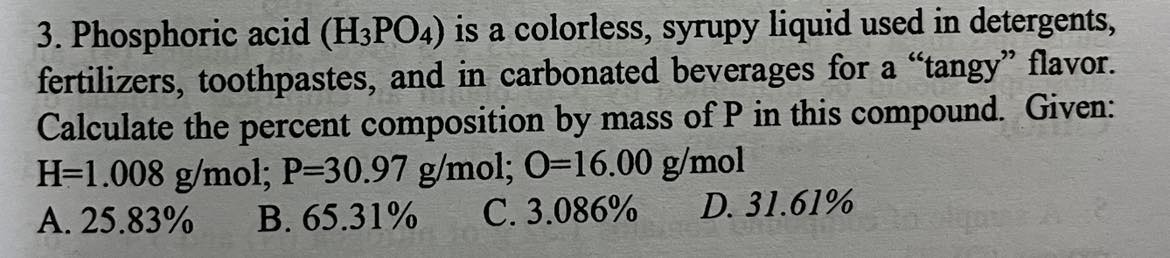
Phosphoric acid (HPO) is a colorless, syrupy liquid used in detergents, fertilizers, toothpastes, and in carbonated beverages for a "tangy" flavor. Calculate the percent composition by mass of P in this compound. Given: H-1.008 g/mol; P=30.97 g/mol; 0-16.00 g/mol
31.61%
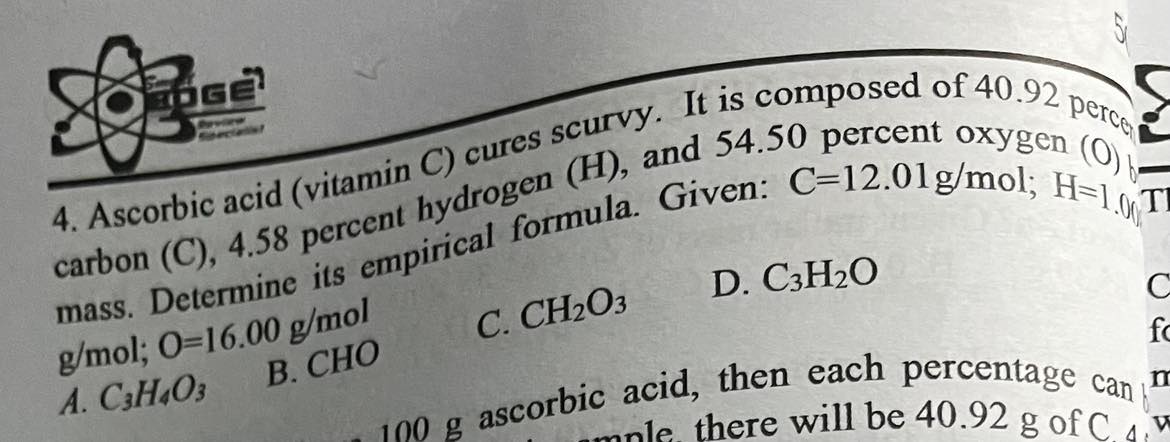
Ascorbic acid (vitamin C) cures scurvy. It is composed of 40.94 perce carbon (C), 4.58 percent hydrogen (H), and 54.50 percent oxygen (O) by mass. Determine its empirical formula. Given: C=12.01g/mol; H=1.008 g/mol; 0=16.00 g/mol
C_3H_4O_3

A sample of compound contains 1.52 g of nitrogen and 3.47 g of oxygen (O). The molar mass of this compound is between 90 g and 95 g. Determine the molecular formula of the compound. Given: N=14.01 g/mol 0=16.00 g/mol
N_2O_4
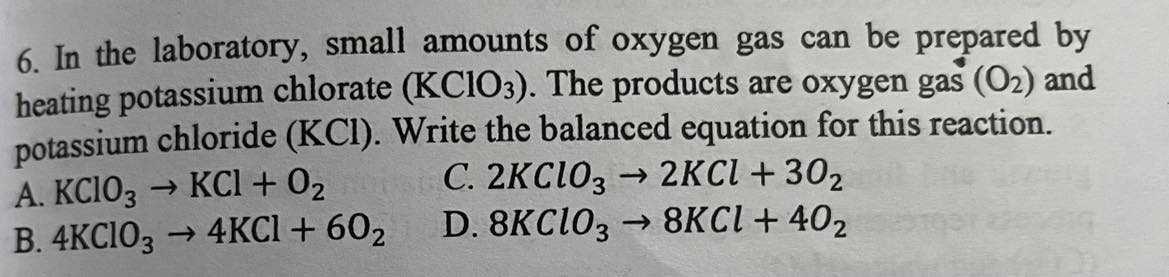
In the laboratory, small amounts of oxygen gas can be prepared by heating potassium chlorate (KCIO). The products are oxygen gas (O2) and potassium chloride (KCI). Write the balanced equation for this reaction.
2KClO_3 —> 2KCl + 30_2
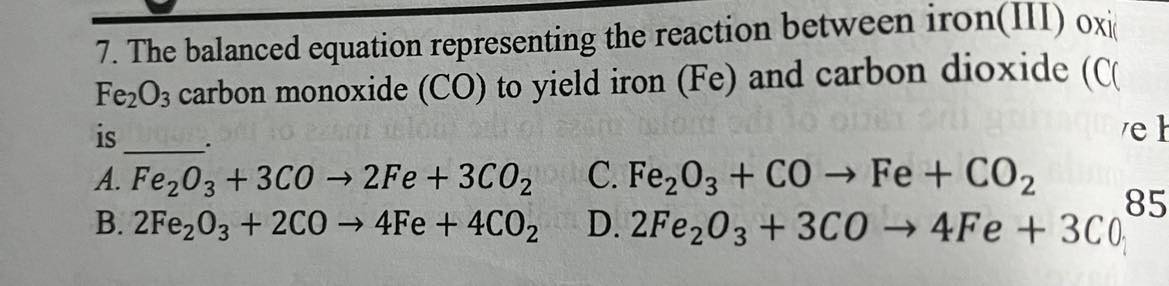
The balanced equation representing the reaction between iron(III) oxide Fe_20_3 carbon monoxide (CO) to yield iron (Fe) and carbon dioxide (CO_2) is
Fe_2O_3+3CO—>2Fe+3CO_2
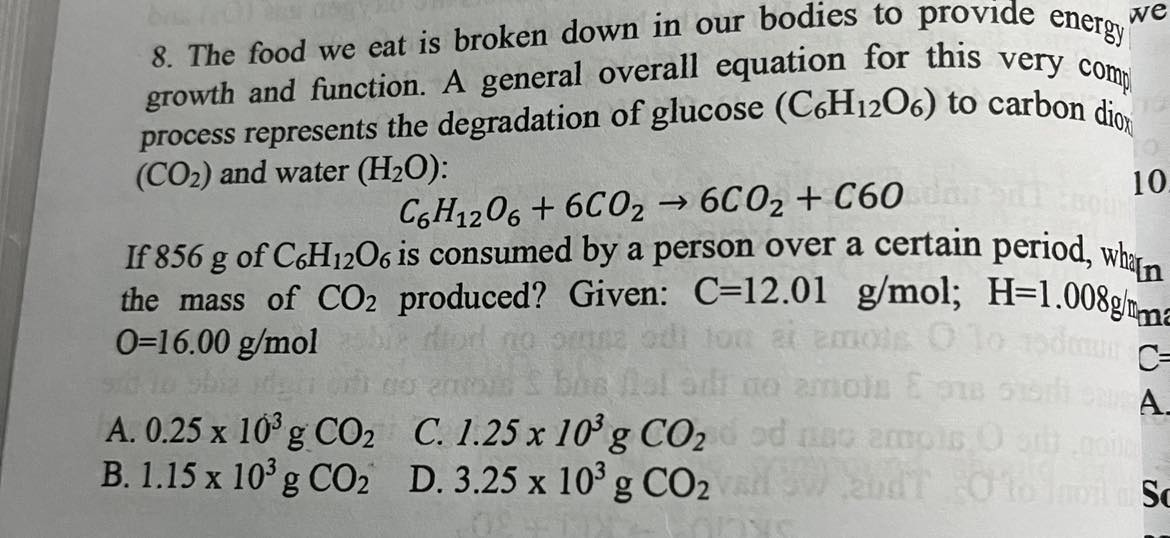
The food we eat is broken down in our bodies to provide energy for growth and function. A general overall equation for this very complex process represents the degradation of glucose (C_6H_120_6) to carbon dioxide (CO2) and water (H2O):
1.25×10³ g CO_2
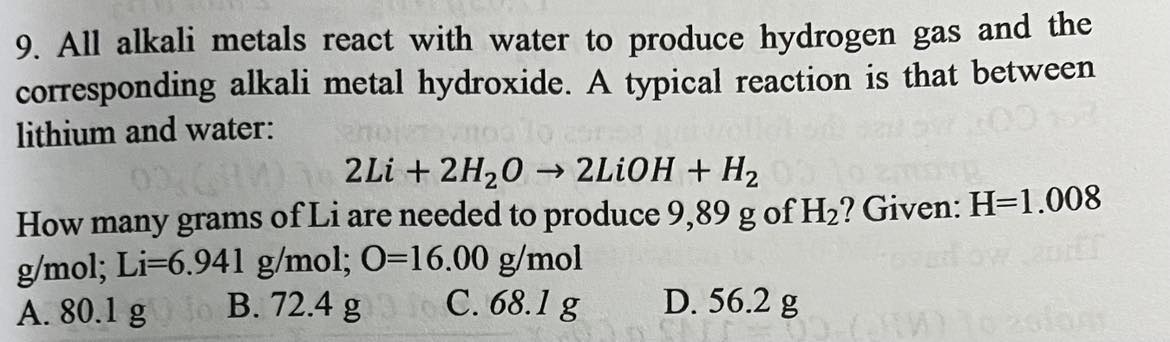
68.1 g Li
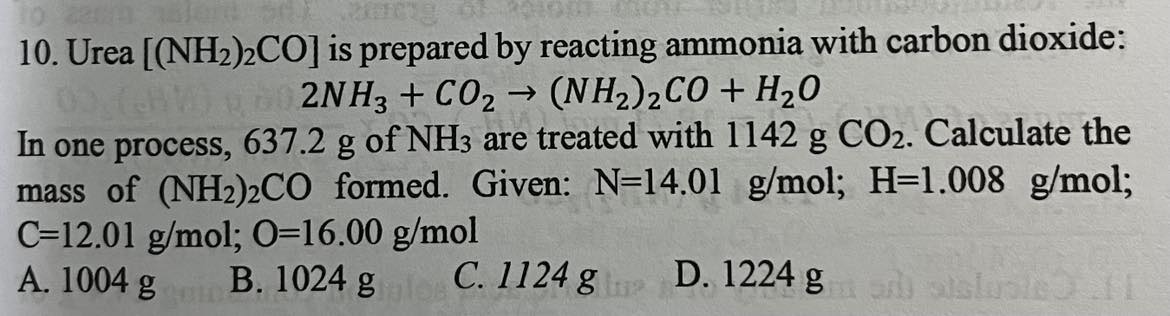
1124 g (NH_2)_2 CO