Chemistry - S1.2 (the nuclear atom)
1/3
Earn XP
Description and Tags
glorp
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
relative mass and charge of subatomic particles (table)
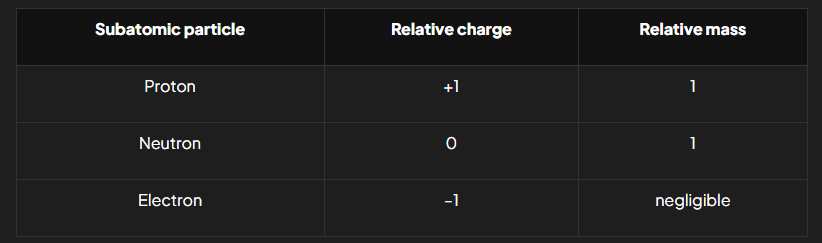
Subatomic particles
protons, neutrons and electrons
so small that it is not practical to measure their masses and charges using conventional units (relative atomic masses’ and ‘relative atomic charges are used instead) Located in the nucleus.
nucleus dense because mass of atom is concentrated in nucleus, which contains heaviest subatomic particles.
subatomic particles in nucleus can be called nucleons (neutrons and protons)
The electrostatic attraction between the positive nucleus and negatively charged electrons orbiting around it is what holds an atom together
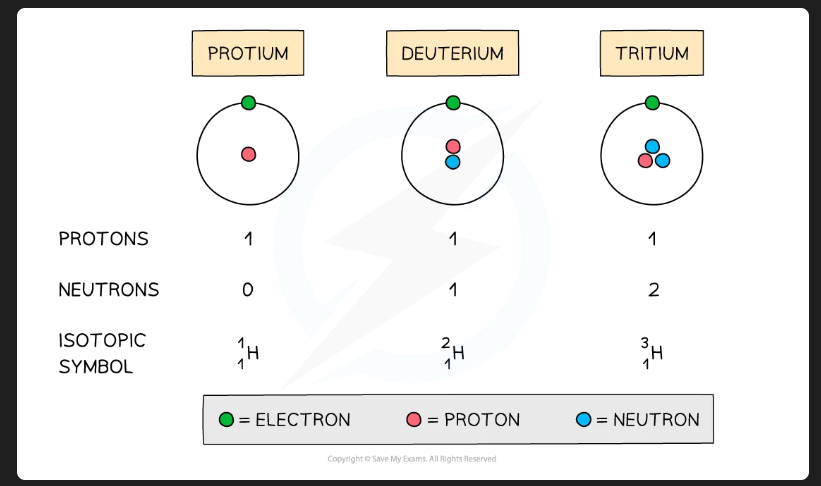
Isotopes
Isotopes = different atoms of same element that contain same number of protons + electrons but different number of neutrons
atoms of same elements but with different mass numbers
Isotopes of hydrogen