Batteries (cells)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What type of cells are rechargable cells?
Secondary
What type of cells are non-rechargeable?
Primary
What are the pros and cons of non-rechargeable cells?
Pros: cheap
Cons: waste issues
What are the pros and cons of rechargeable cells?
Pros: less waste, cheaper in the long run, lower environment impact
Cons: Some waste issues (at end of useful life)
What type of cell is the lithium battery?
Storage cells (batteries)
What is the reaction that occurs at the negative electrode in the lithium ion battery?

What is the reaction that occurs at the positive electrode in the lithium ion battery?
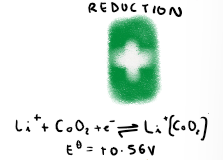
What is the overall equation for the lithium ion battery?

Why are secondary cells rechargeable?
What are the pros and cons of using hydrogen fuel cells?
Pros: only waste product is waster, do not need re-charging, very efficient
Cons: needs constant supply of fuels, hydrogen is flammable and explosive, hydrogen usually made using fossil fuels
High cost of fossil fuels
What typeof hydrogen fuel cells are there?
Acid and alkaline
What is the reaction at the negative electrode in alkaline hydrogen fuel cells?
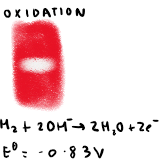
What is the reaction at the positive electrode in alkaline hydrogen fuel cells?
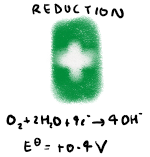
What is the overall reaction of alkaline hydrogen fuel cells?
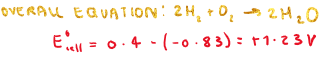
What is the reaction at the negative electrode in acid hydrogen fuel cells?
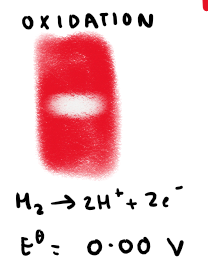
What is the reaction at the positive electrode in acid hydrogen fuel cells?

What is the overall equation of acid hydrogen fuel cells?

What can be used to fuel H+ ions in the acid hydrogen fuel cell?
Alcohol can be used as fuel to supply H+ ions (alcohol can be oxidised at anode with water present)
These H+ ions could then be oxidised to water after they pass through an electrolyte