L11: Glucose Regulation Pt. 1
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
102 Terms
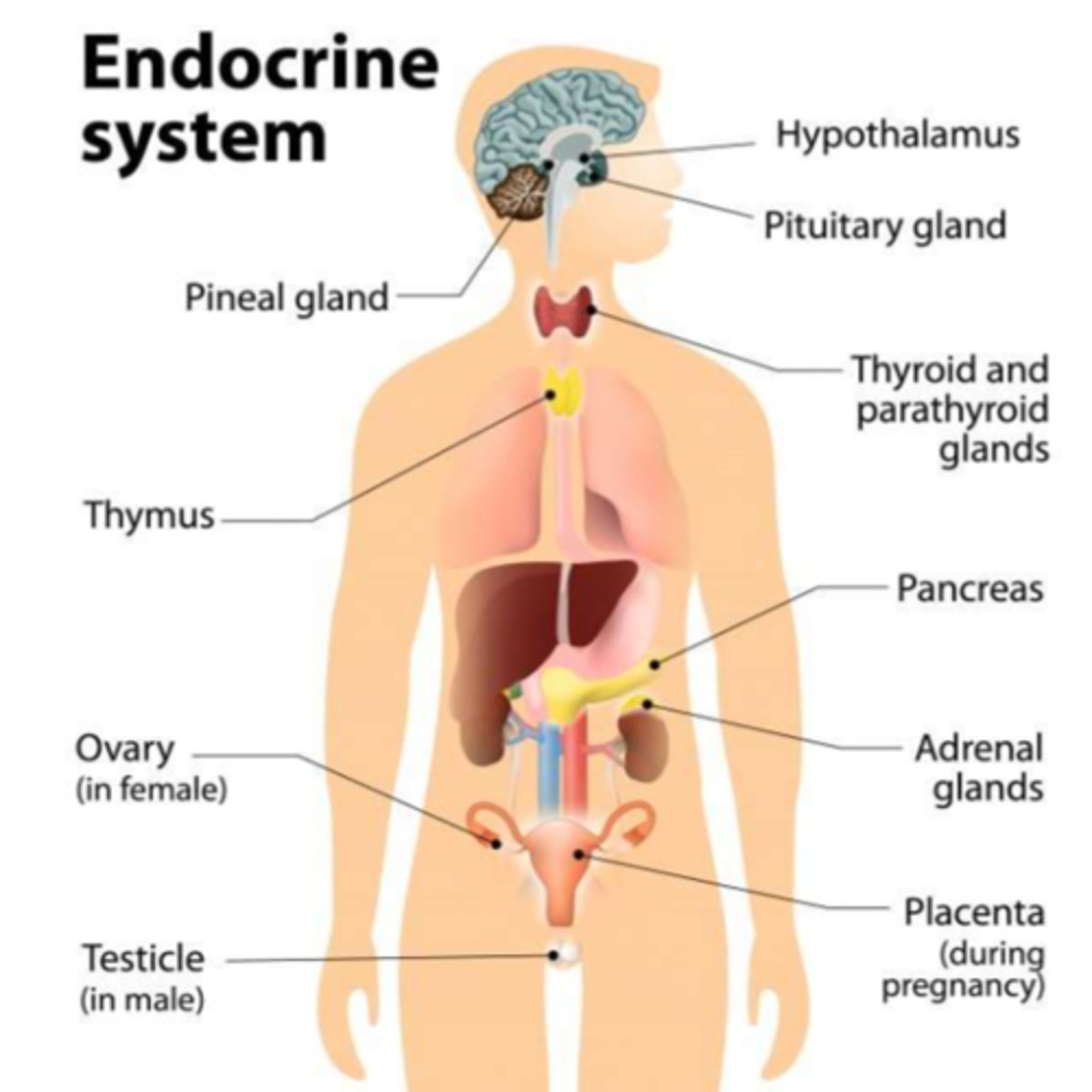
Where does glucose & hormone regulation occur?
Endocrine system
What are all sugars broken down into as a final product?
Glucose (aka dextrose - synonymous term)

What different sugars can be broken down into glucose?
- fructose: sugar in fruit
- maltose: sugar in malt/beer
- lactose - sugar in milk

How are splenda & aspartame used as a sugar substitute?
- travel unchanged, will not be broken down just end up excreted
- molecules are so small that they are basically 0 cals (aspartame are in 0 cal drinks)

What are endocrine organs responsible for?
They synthesize & secrete 'hormones' (aka "endocrinology")
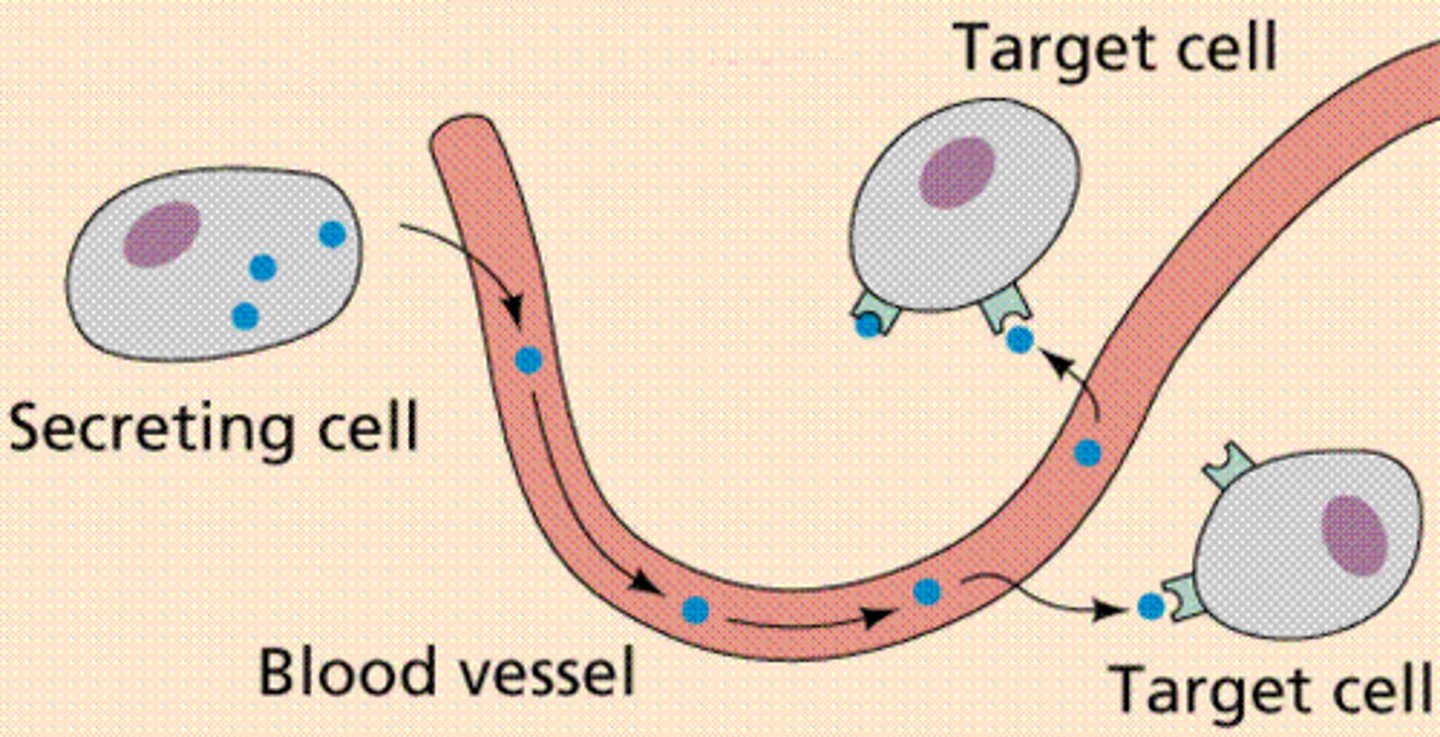
What happens with the endocrine hormones that are secreted?
- Hormones - released into bloodstream
- 'Paracrine' hormones - act on other cells than where they are synthesized
- Long half life = likely highly PPB (eg. T4 hormone)
What is endocrine hormone secretion triggered by?
- Concentration of specific substances (eg. glucose)
- Neural stimulation (eg. SNS & epinephrine)
- Endocrine sequences (eg. epinephrine => aldosterone ...)
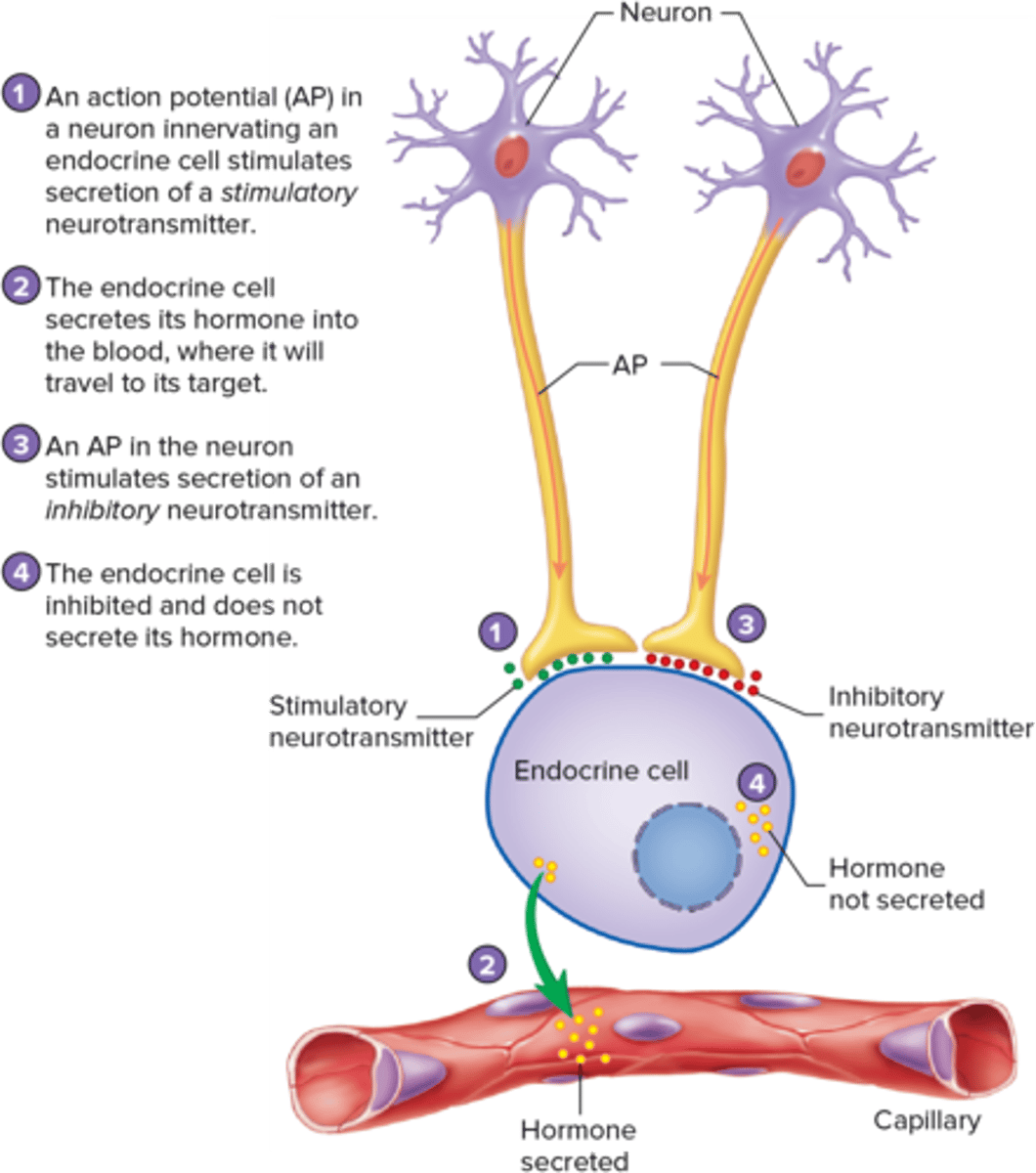
What is endocrine hormone secretion regulated by?
negative feedback mechanisms
How to test for functionality of endocrine hormones?
- through function tests: hormone level or the effector-substance level
- eg. glucose (indirect test of insulin function); TSH, ...
What are the main endocrine dysfunctions & the s&s that will follow?
- hyposecretion or hypersecretion (primarily use BW to measure)
- s&s will be directly related to excess or deficit of the expected hormone level
What are endocrine dysfunctions caused by? (hypo vs hyper)
- overall: primary endocrine disorder, signaling disorder, sequence disorder
- hyposecretion: congenital defect, disease/infection/inflammation, hypoperfusion, ageing
- hypersecretion: genetic, tumors, environmental stimuli
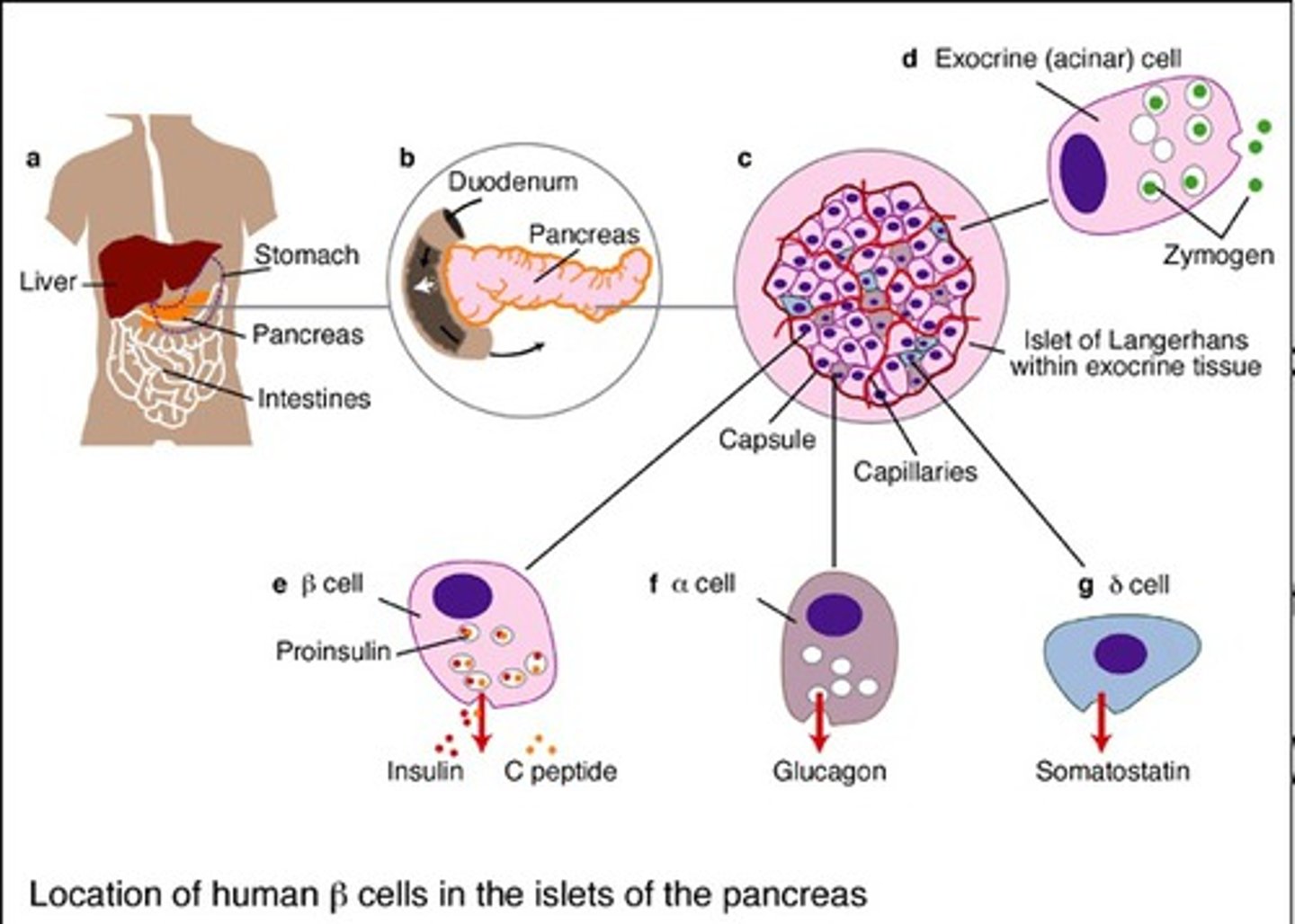
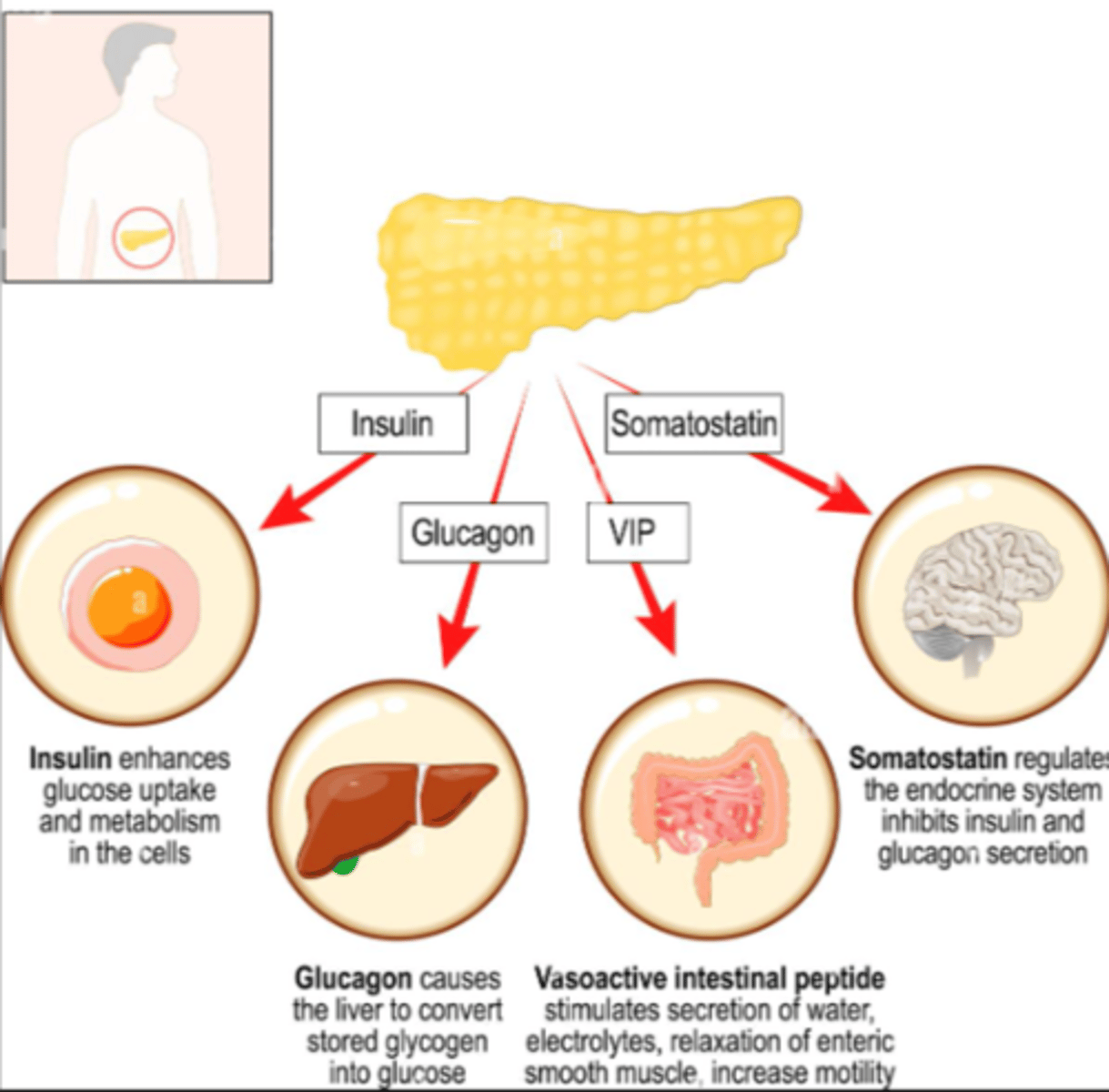
What is somatostatin?
- inhibitory hormone
- seen in glucose regulation
- "shut off trigger" for inhibiting glucagon & insulin as needed
What are our primary energy sources?
- 1st: glucose (broken down immediately in GI & readily distributed)
- 2nd: fatty acids (fat)

How is the CNS involved in glucose use?
- CNS (brain) = most 'needy' system
- requires constant supply of glucose (broken down: CO2 & H2O)
- Cannot store it for later
What happens with extra glucose in the body?
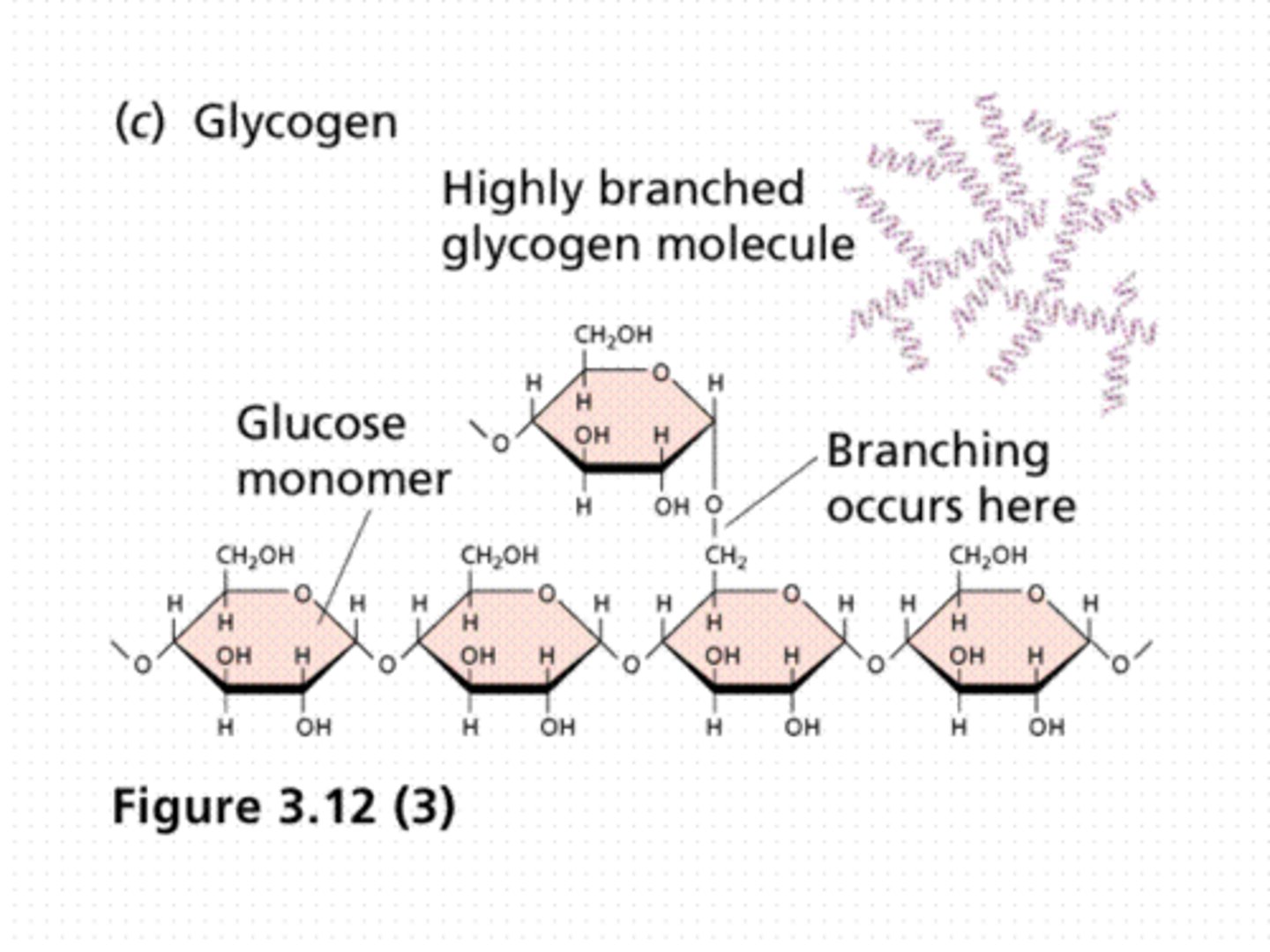
stored as glycogen (liver, muscles) & triglycerides (adipose cell)
What happens if there is a fall in blood glucose?
- glycogen breakdown via 'glycogenolysis'
- there will also be formation of more glucose from other sources ('gluconeogenesis') and released PRN
What is glycogen?
glucose stores that are stacked up to be used as necessary reserves
How are fatty acids used in the body
- distributed via lymph to circulation
- CNS & RBCs cannot use fatty acids (and rely on non-insulin-dependent glucose transporters for glucose uptake)
- extra FAs: stored as triglycerides => broken-down into: 3 fatty acids & glycerol (glycolytic pathway into glucose)
- fatty acids are NOT converted into glucose => cannot be used by the brain for energy
- fatty acid metabolism in liver => ketone metabolites (always present & used for energy)
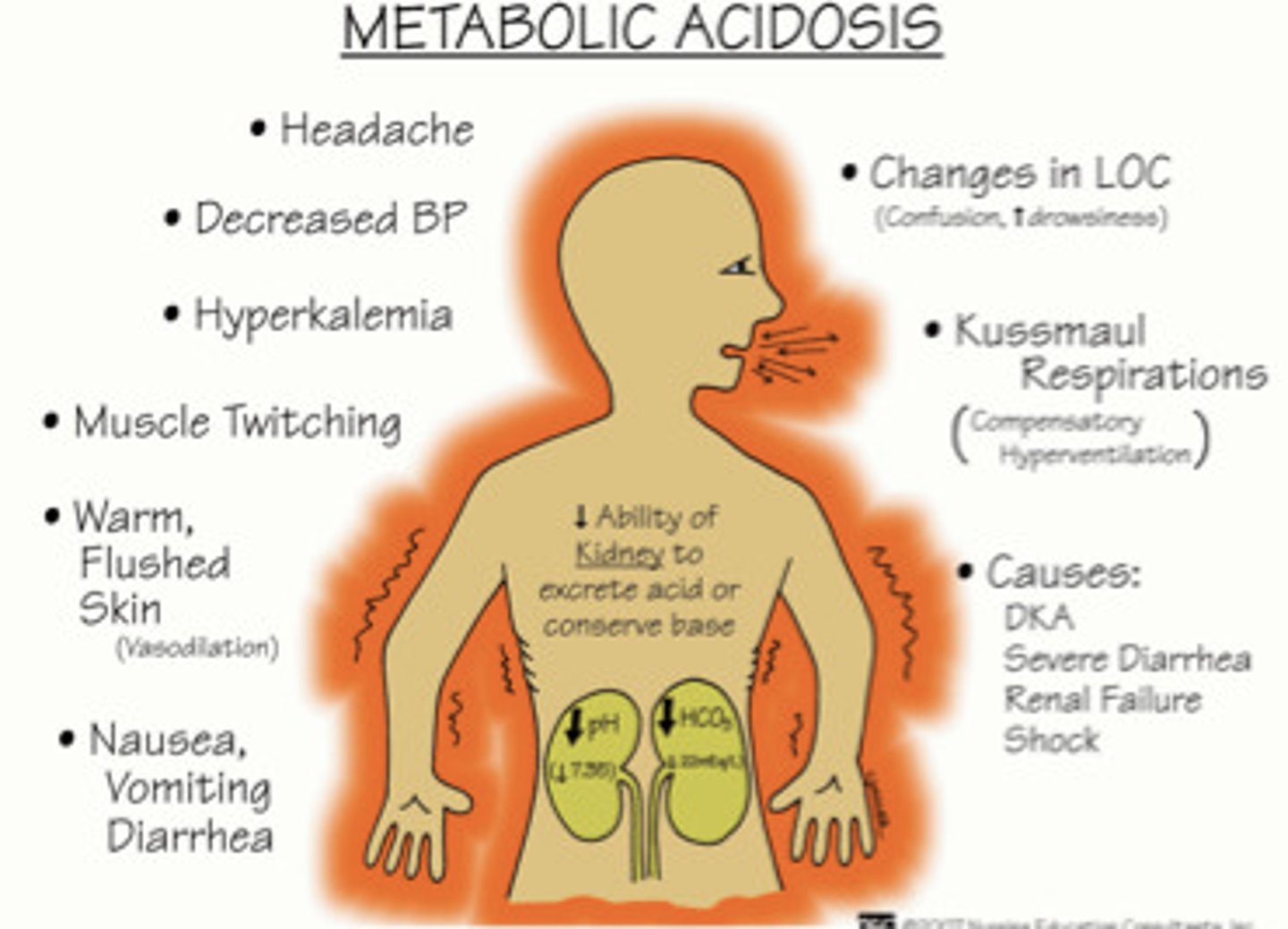
What happens if there are high amounts of ketone metabolites?
- causes metabolic acidosis & glucose is not readily available
- too many fatty acids are being broken down, must test for this - bad!
- increases anaerobic metabolism & lactate presence
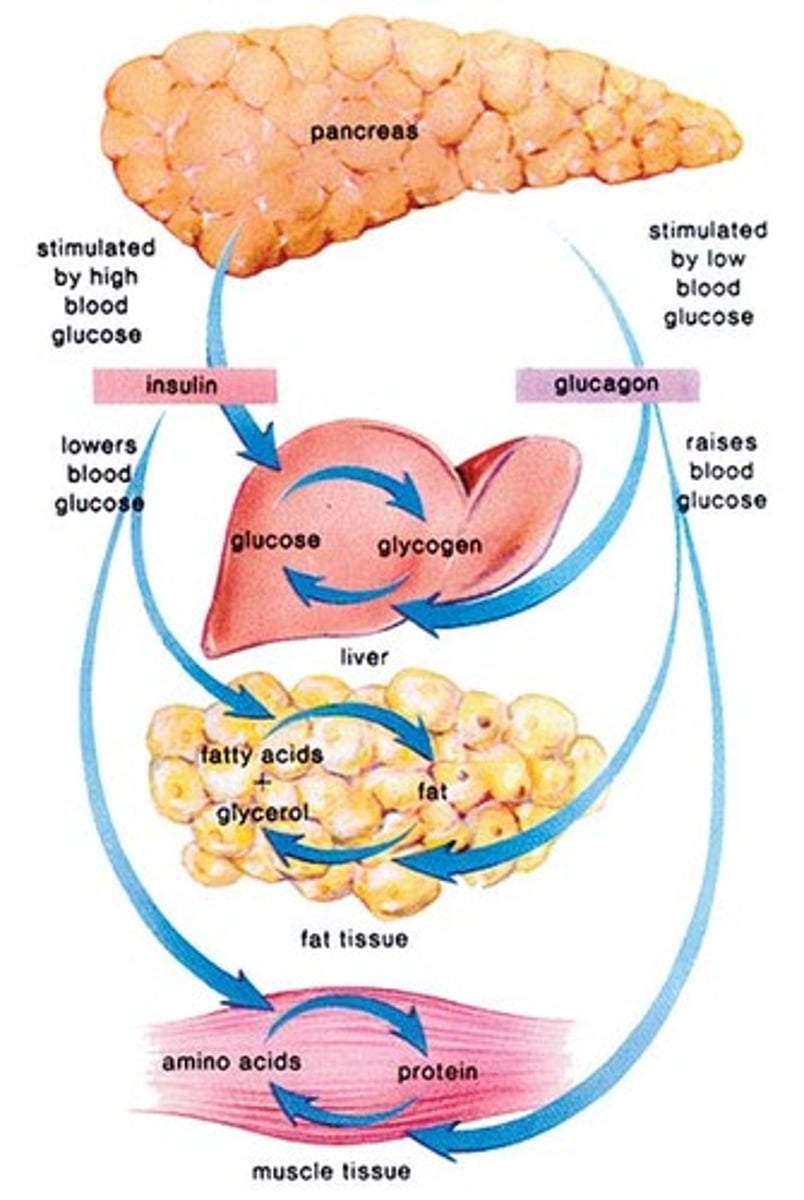
What is insulin?
- pancreatic endogenous hormone that provides energy via blood sugar and glucose in cells
- unlocks entry for glucose energy entry into cell
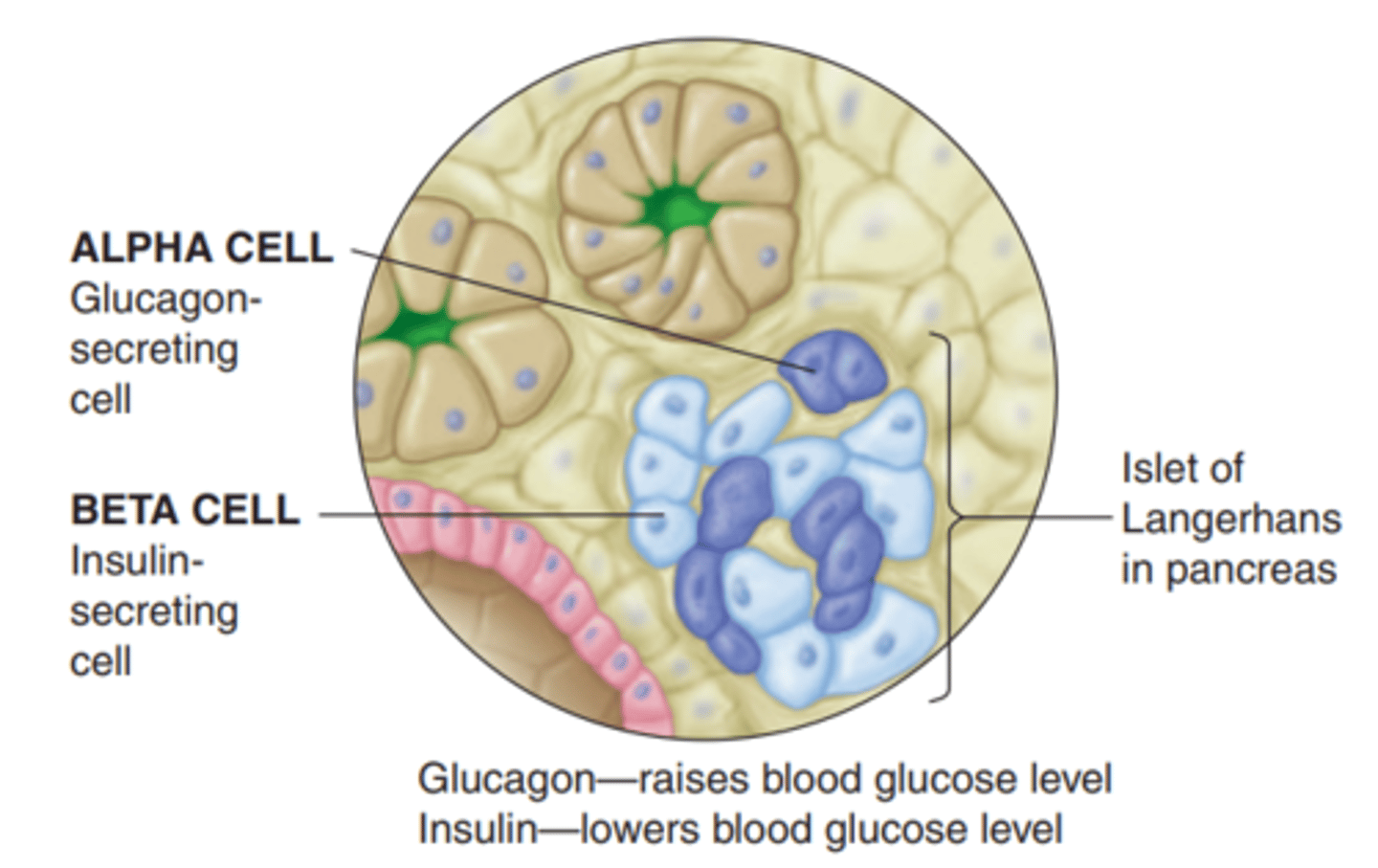
- produced in pancreas, synthesized in beta cells (Langerhans)
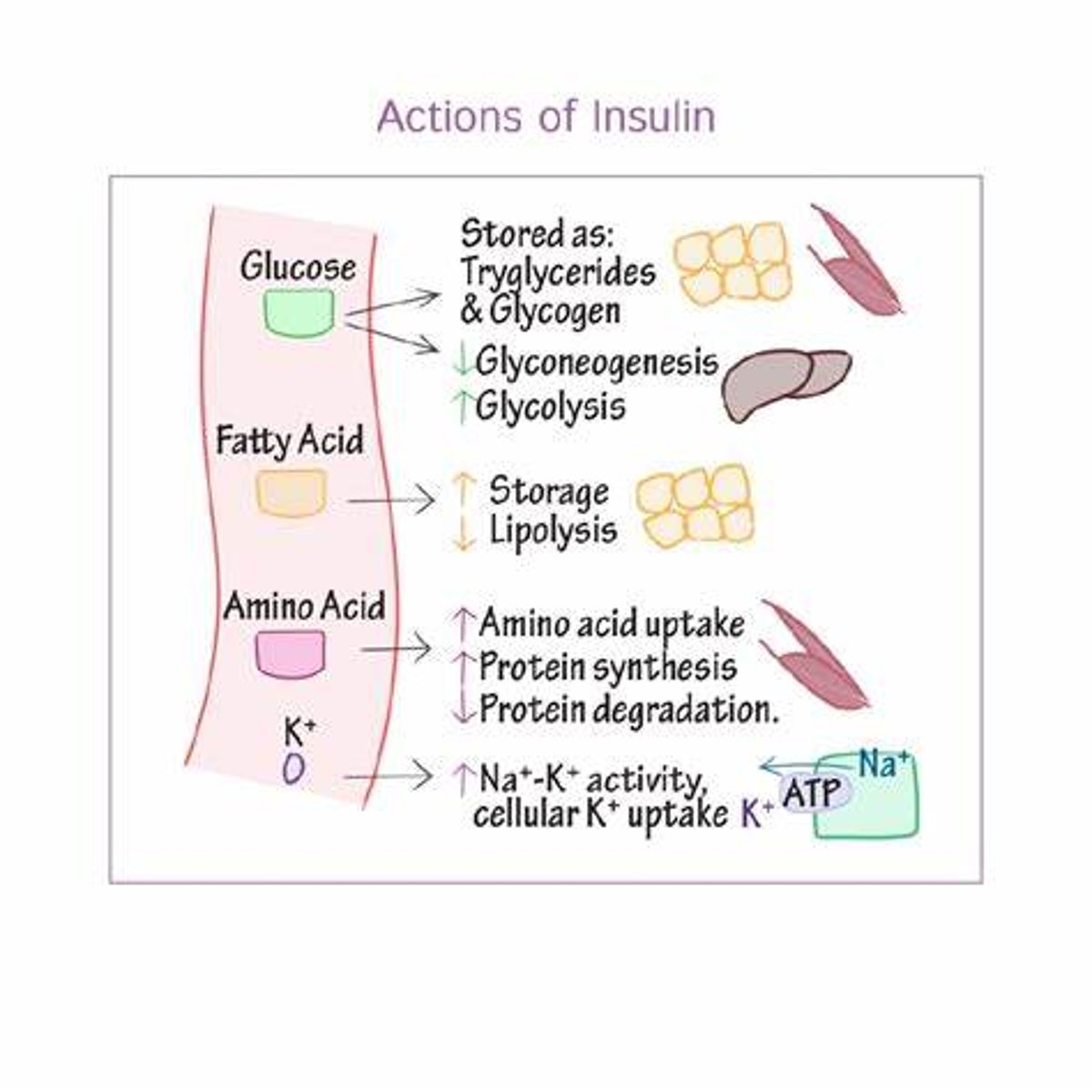
What are the main actions of insulin?
- cellular uptake of glucose
- promotes storage formation (glycogen synthesis, triglyceride synthesis, protein synthesis) (need to store glucose in hepatocytes) => prevents glycogen & fat lysis (in order to 1st use glucose) & protein lysis (to preserve tissues)
- amino acid cellular uptake; triglyceride adipose cell uptake
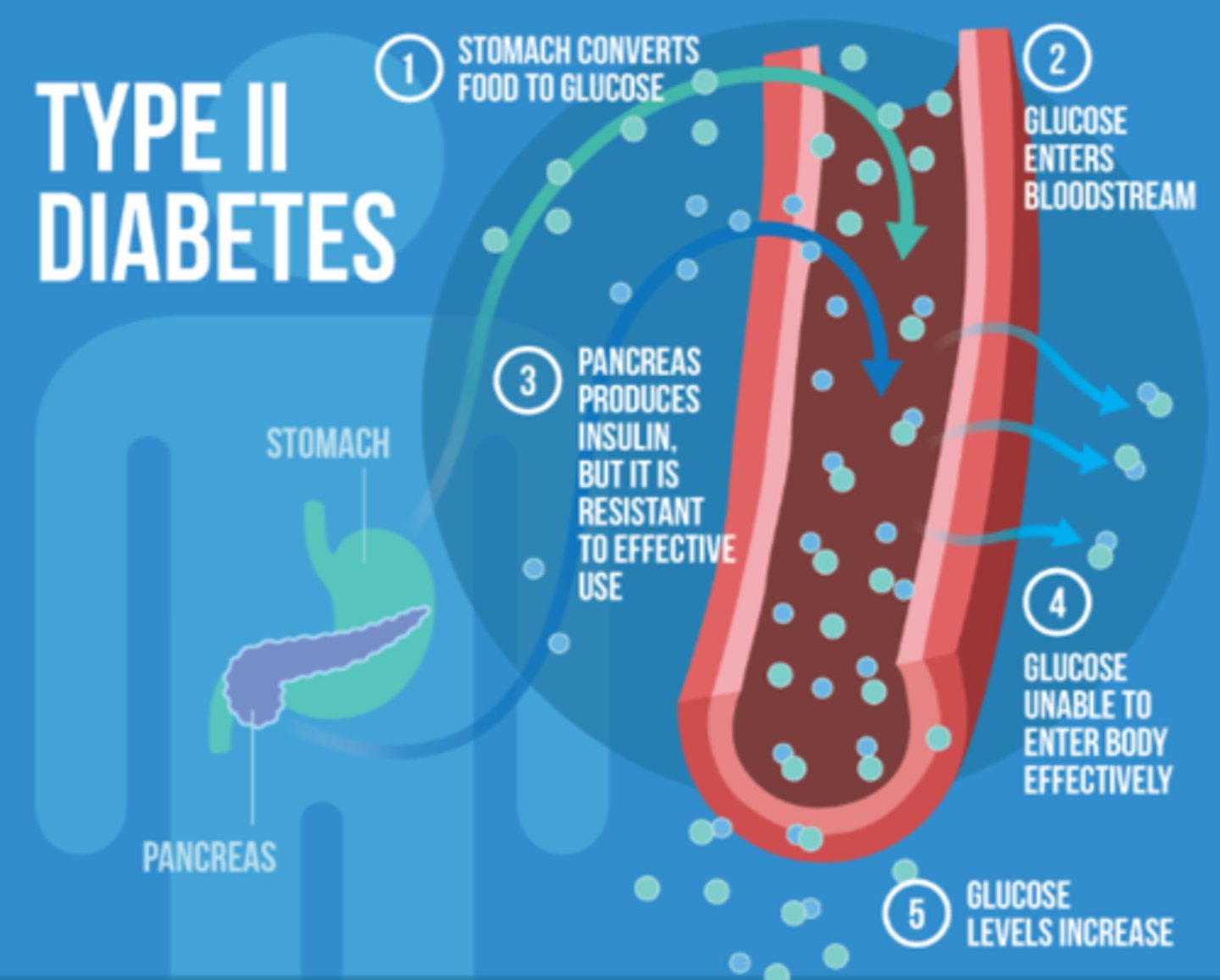
What happens with insulin in type 2 diabetes?
- cannot use pancreas as well & cells stay locked
- insulin resistance may develop & glucose cannot get in, building up in bloodstream
- results in glucose deficiency => BAD! need glucose in cells
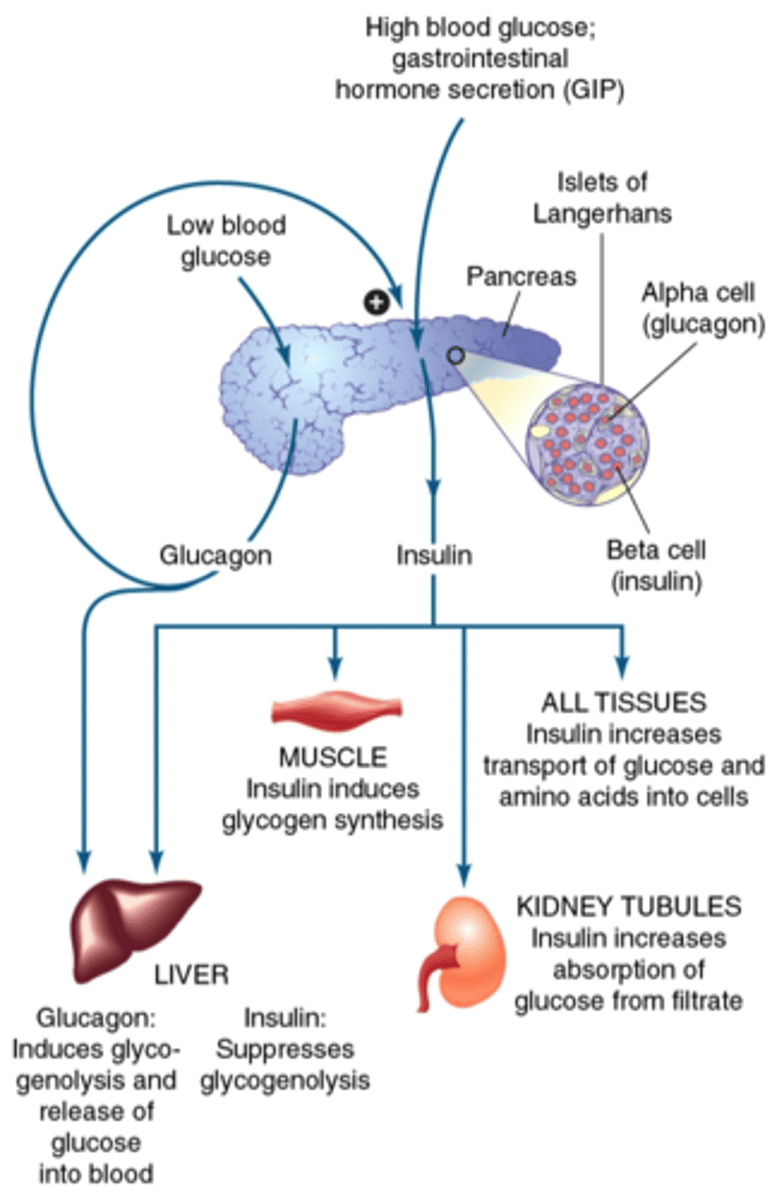
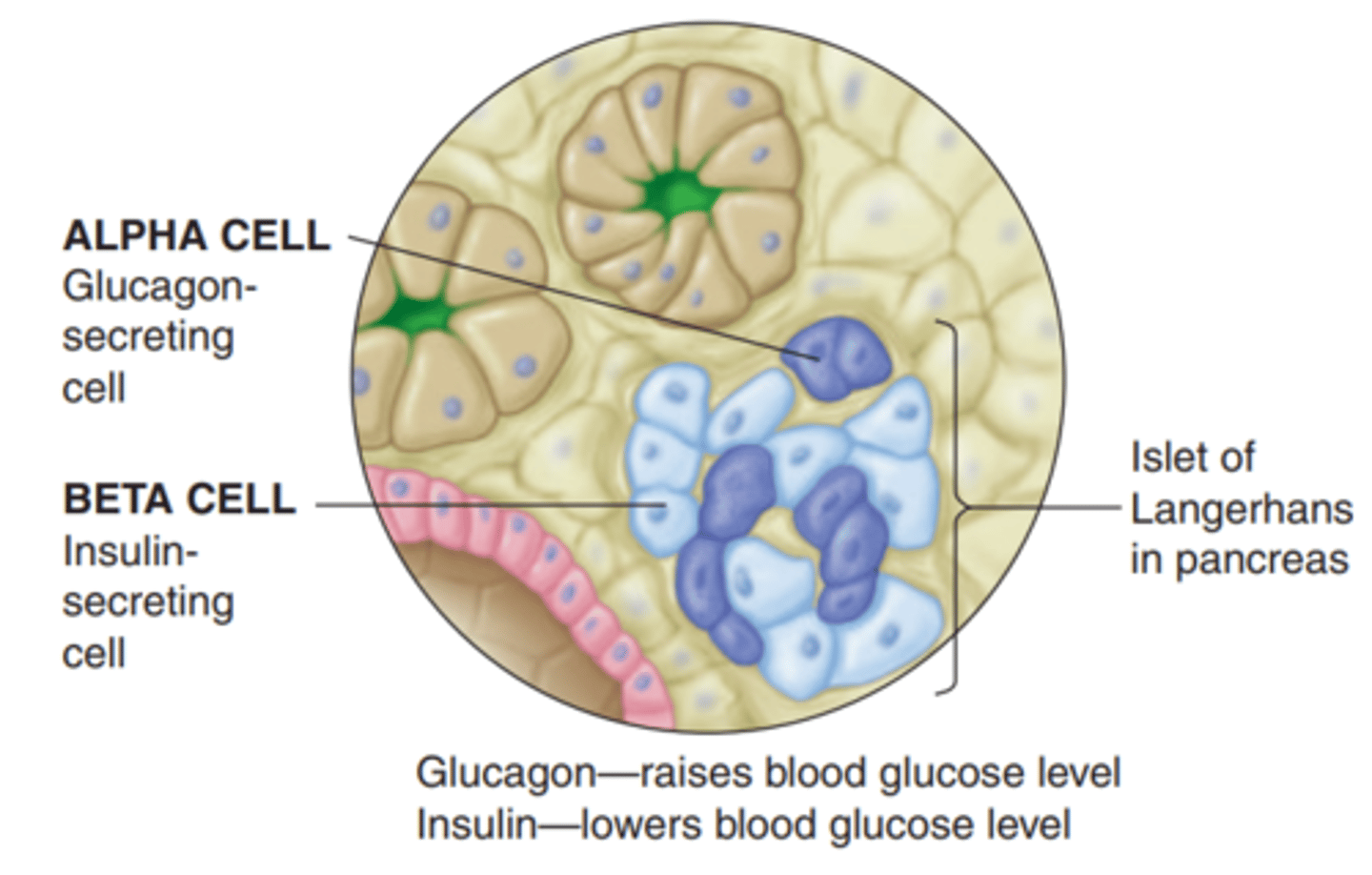
What is glucagon?
- synthesized in Alpha cells = opposite of insulin
- promotes mobilization of stores: glycogenolysis (glycogen breakdown); gluconeogenesis (amino acid conversion into glucose); lipolysis (triglyceride breakdown)
- triggered by low plasma glucose levels (between meals; hypoglycemia) => mobilize stores and replenish blood glucose for cellular use
What is glucosuria and how does it occur?
glucose in urine - happens when blood glucose levels exceed the kidney's reabsorption capacity - eg. hyperglycemia
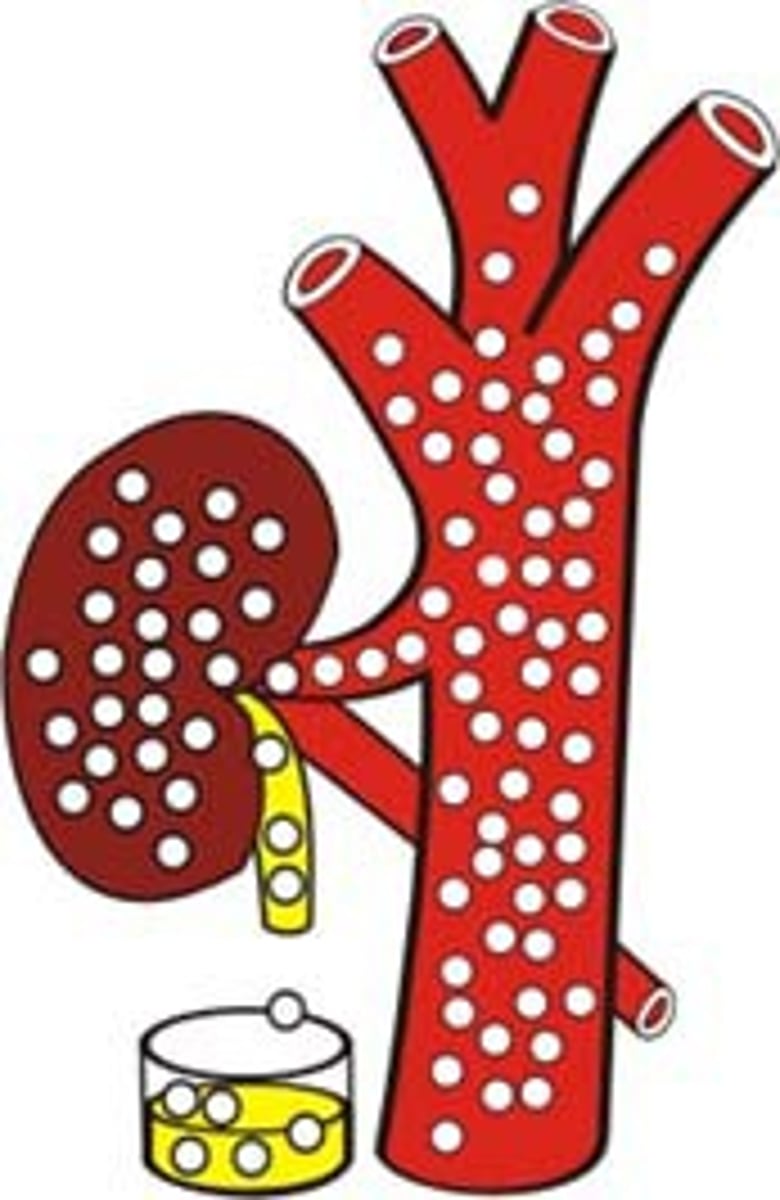
What is glycemic regulation?
- blood glucose regulation
- optimum GI delivery from serum to tissues
- negative feedback loop
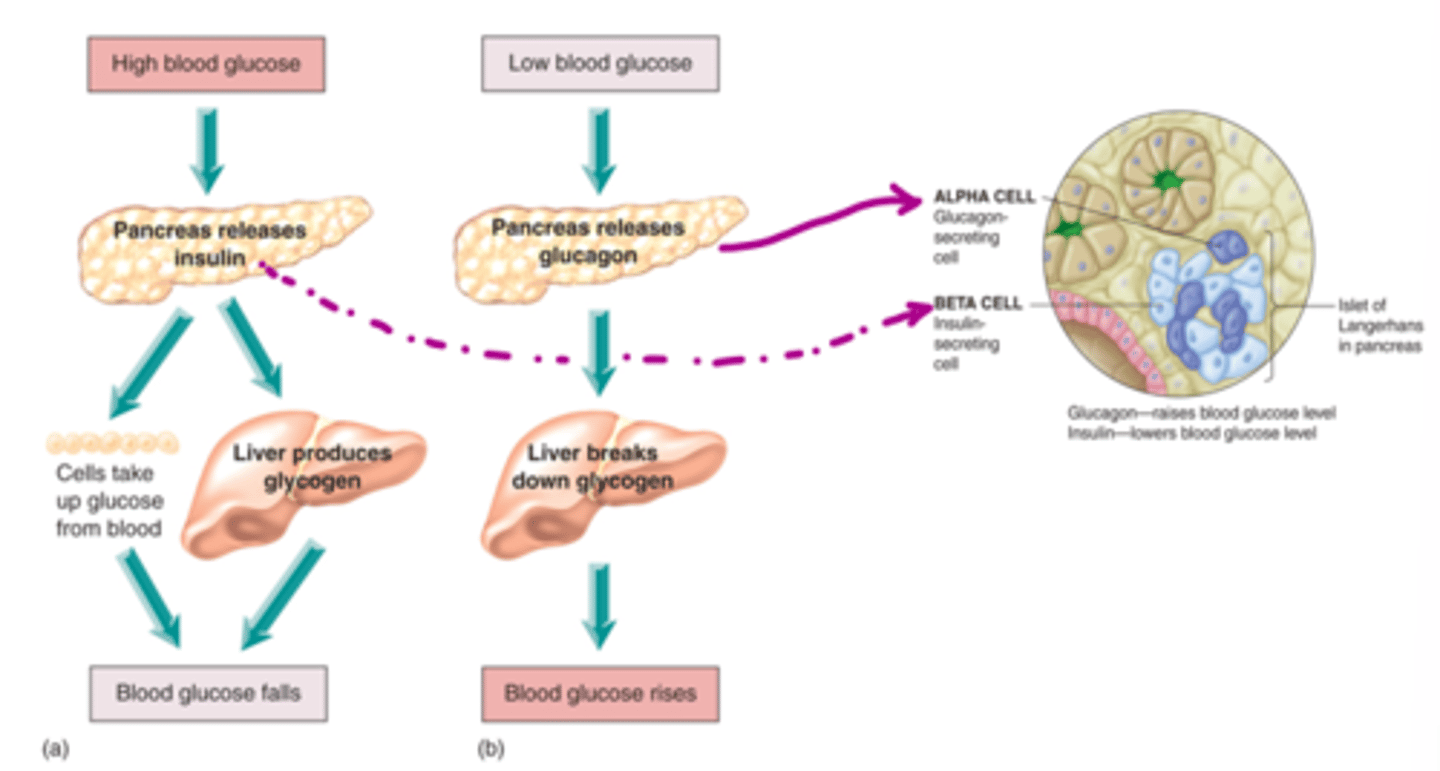
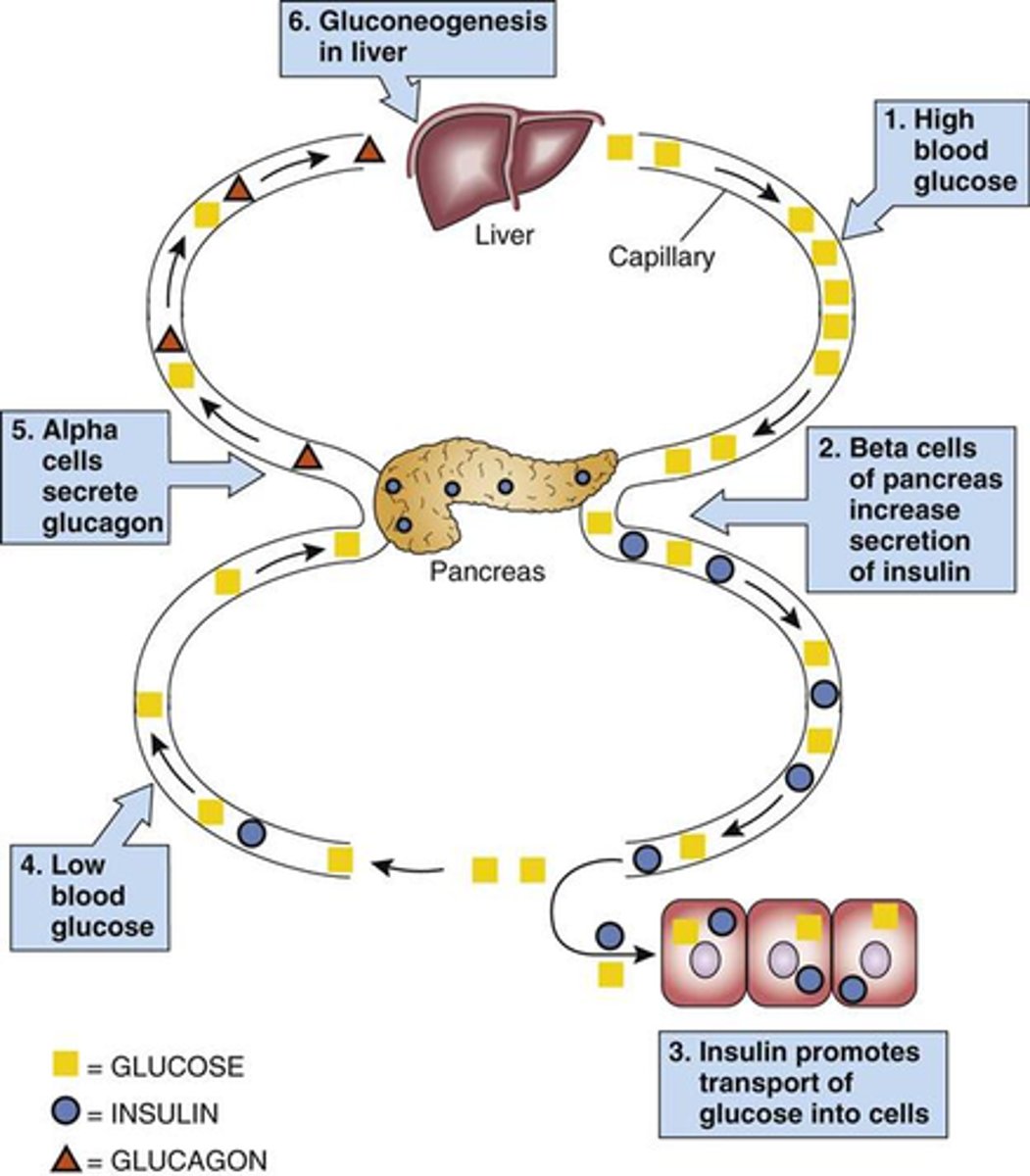
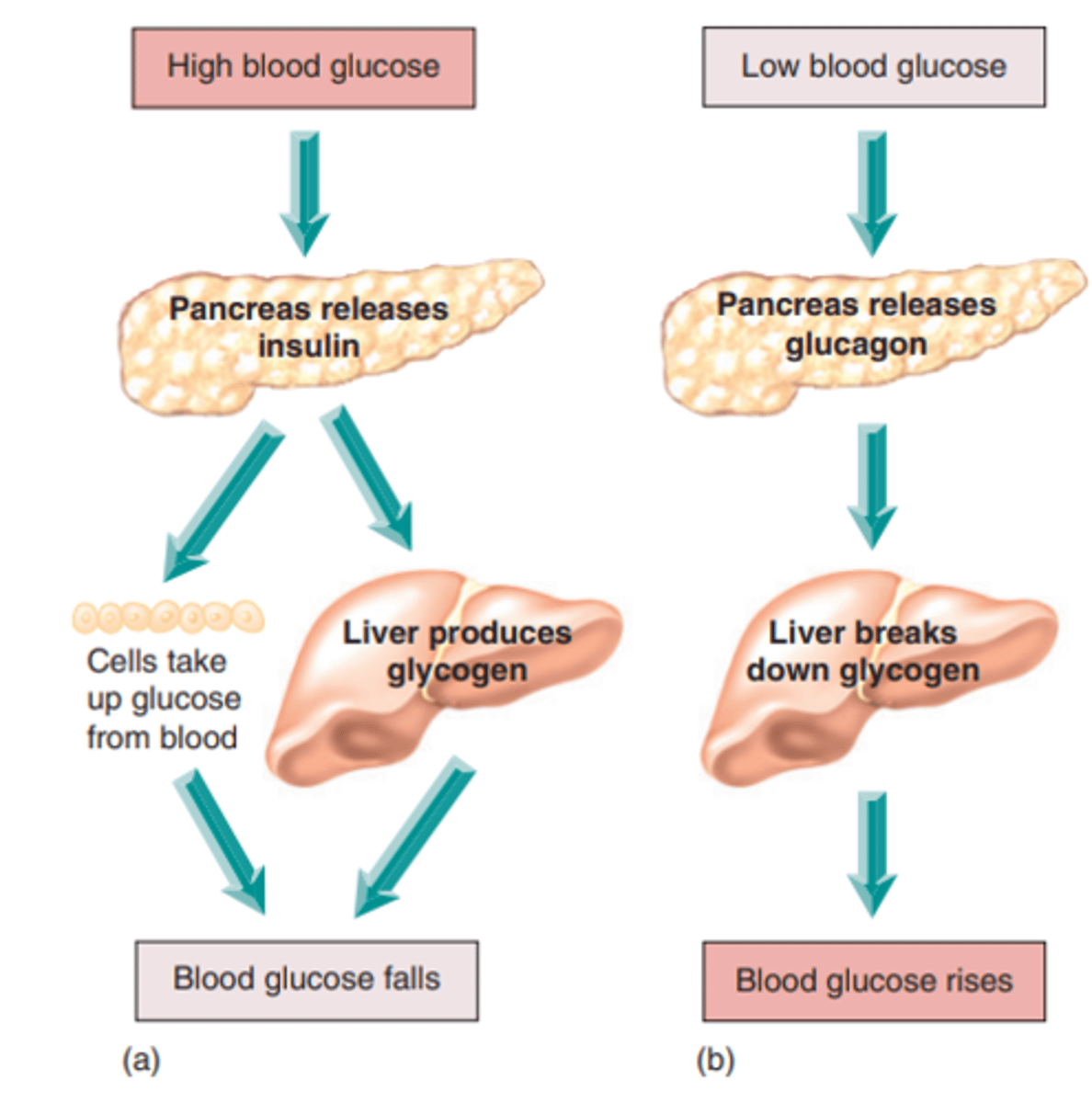
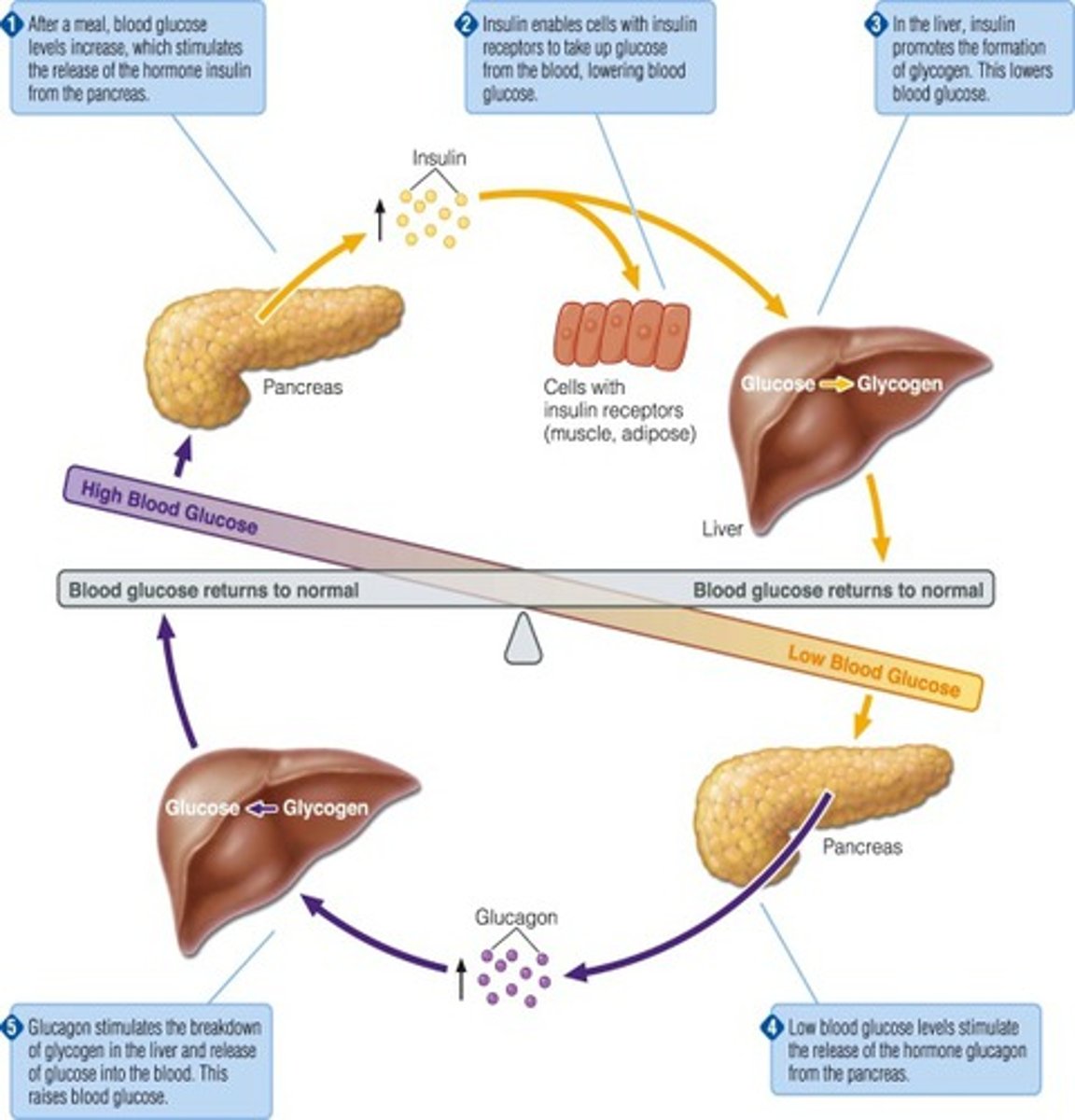
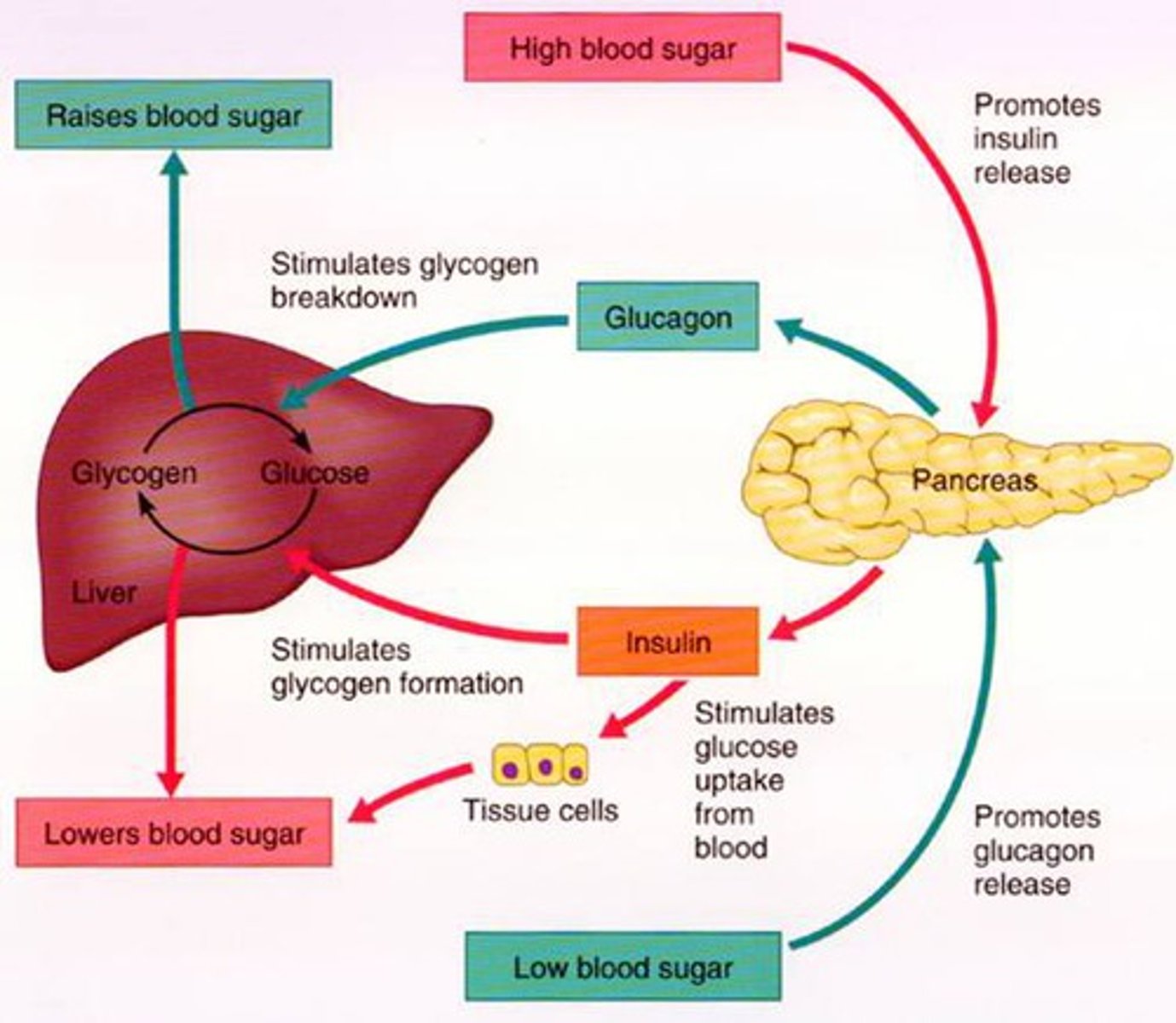
How does the body regulate HIGH blood glucose (negative feedback)
- pancreas releases insulin, secreted by beta cells
- cells take up glucose from blood while liver produces glycogen
- blood glucose levels fall back to normal
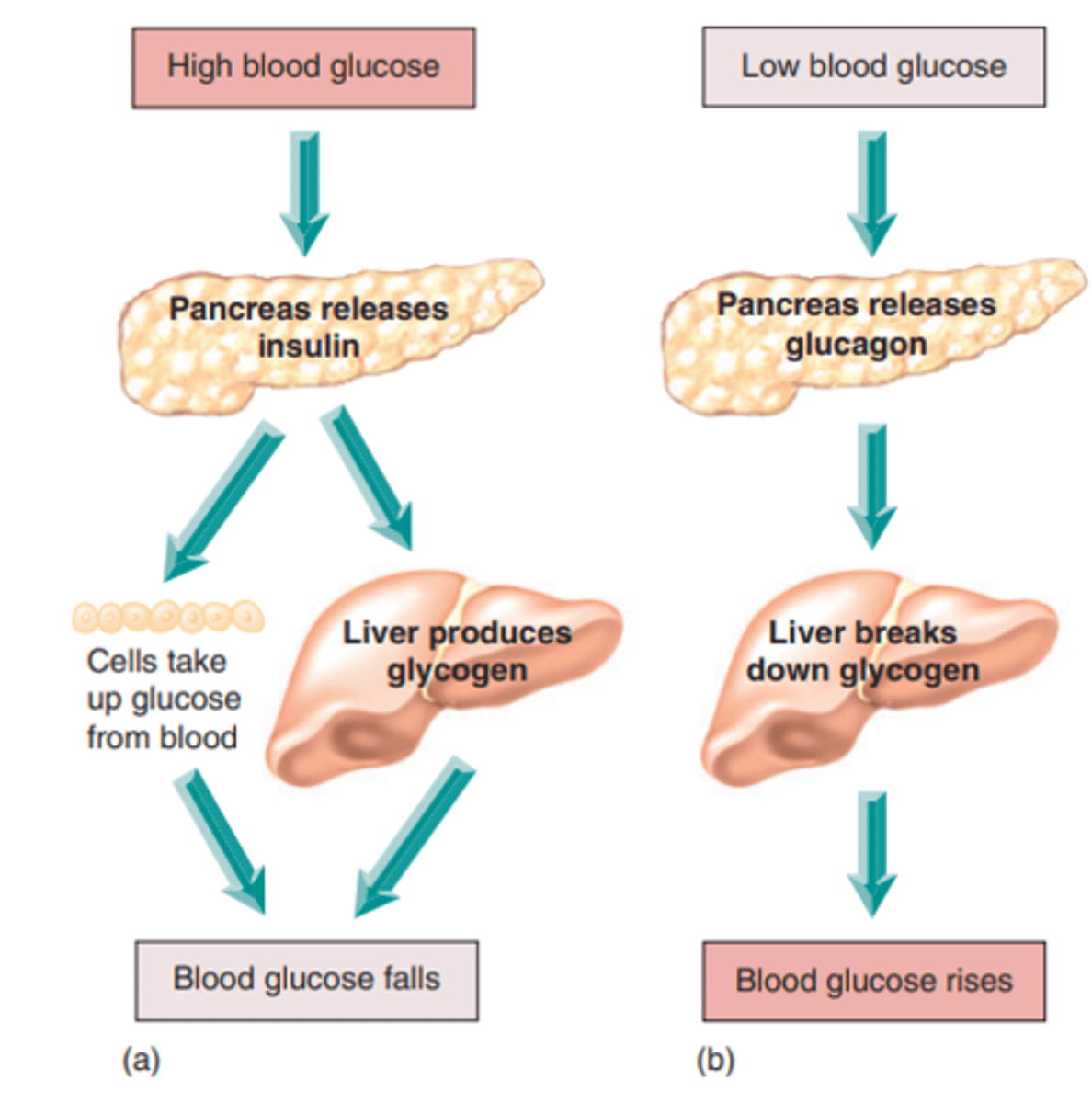
How does the body regulate LOW blood glucose (negative feedback)
- pancreas releases glucagon, secreted by alpha cells
- liver breaks down glycogen
- blood glucose levels rise back to normal
What is the function of beta cells?
- cells that synthesize insulin
- stimulus: high levels of serum glucose
- Glucose enters Pancreatic Beta cell via Glucose transporter => metabolized via Glucokinase into ATP => closes K channels (on Beta cell) => depolarization => Insulin secretion
- Insulin from pancreas enters hepatic circulation => 50% 1st pass metabolism => metabolites are renally excreted
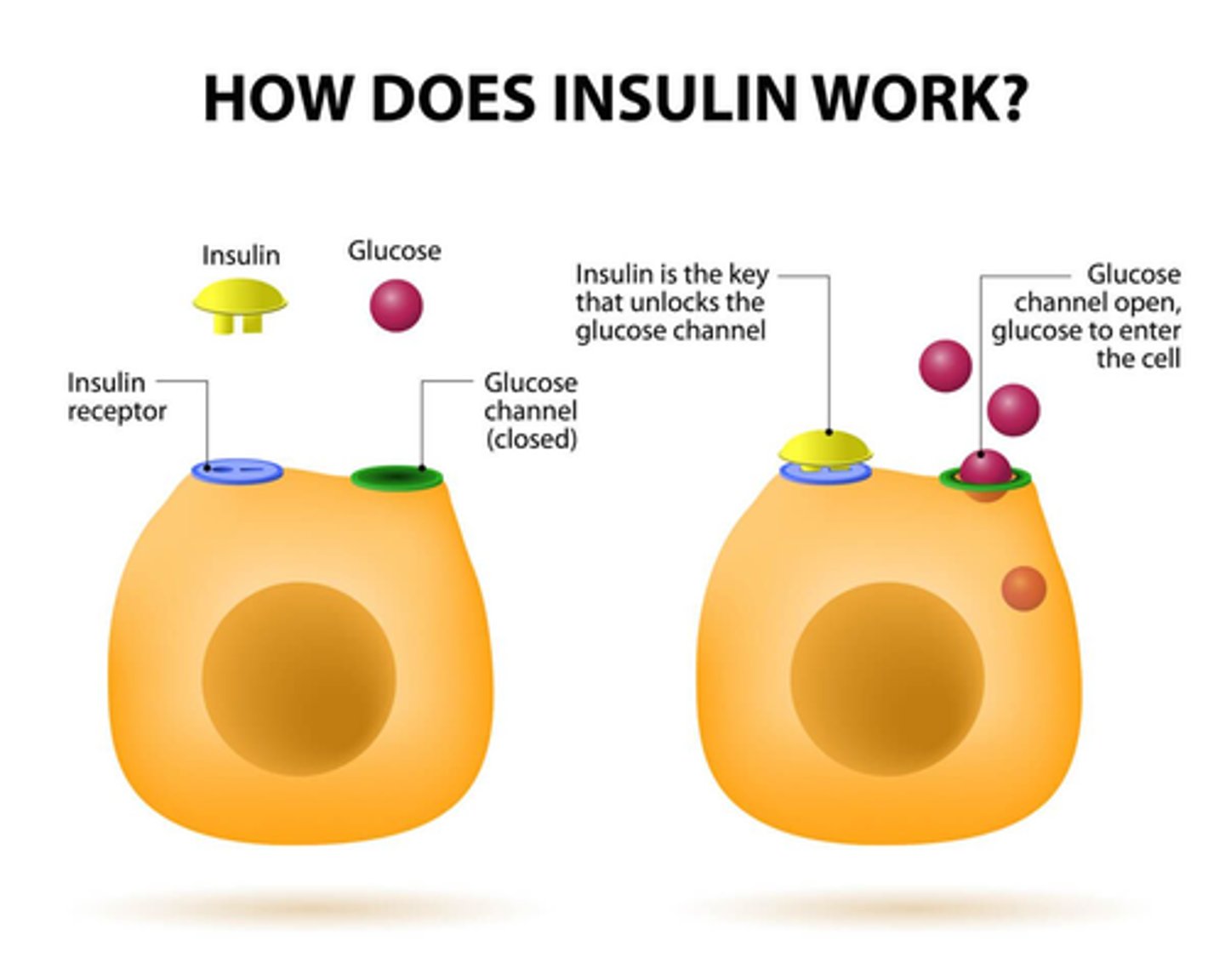
What does insulin do at cellular level?
binds to cellular transmembrane receptor (tyrosine kinase) => activates kinase enzyme within cell => stimulates Glucose transporter channels to open on the surface of the cell for Glucose
What hormone is responsible for insulin inhibition?
Somatostatin (D cell produced)
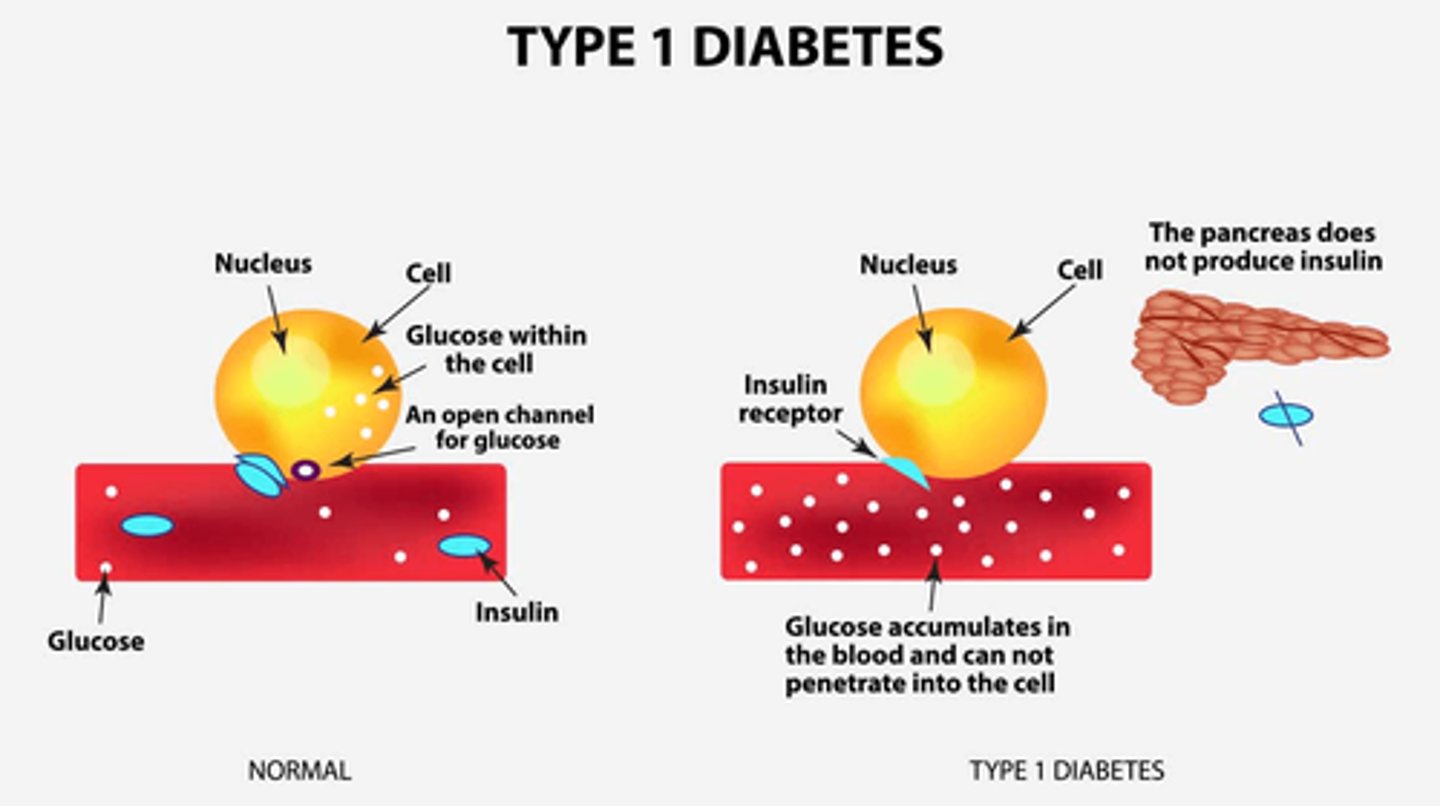
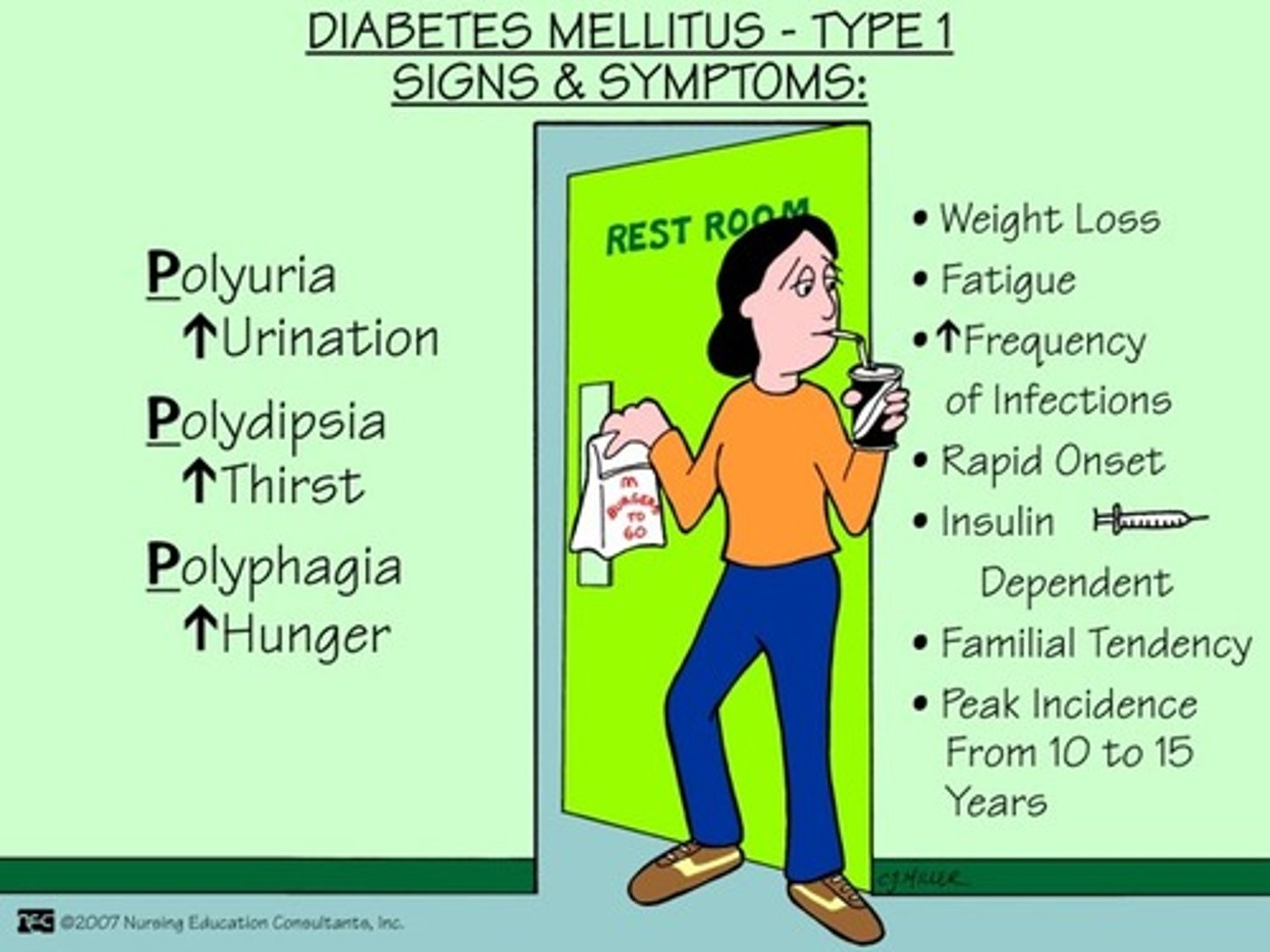
What is diabetes type 1 (DM I)
- destruction of beta cells!!!
- dysfunction of glucose, fat, and protein metabolism
- immediate effects: disabled transport of glucose into cells
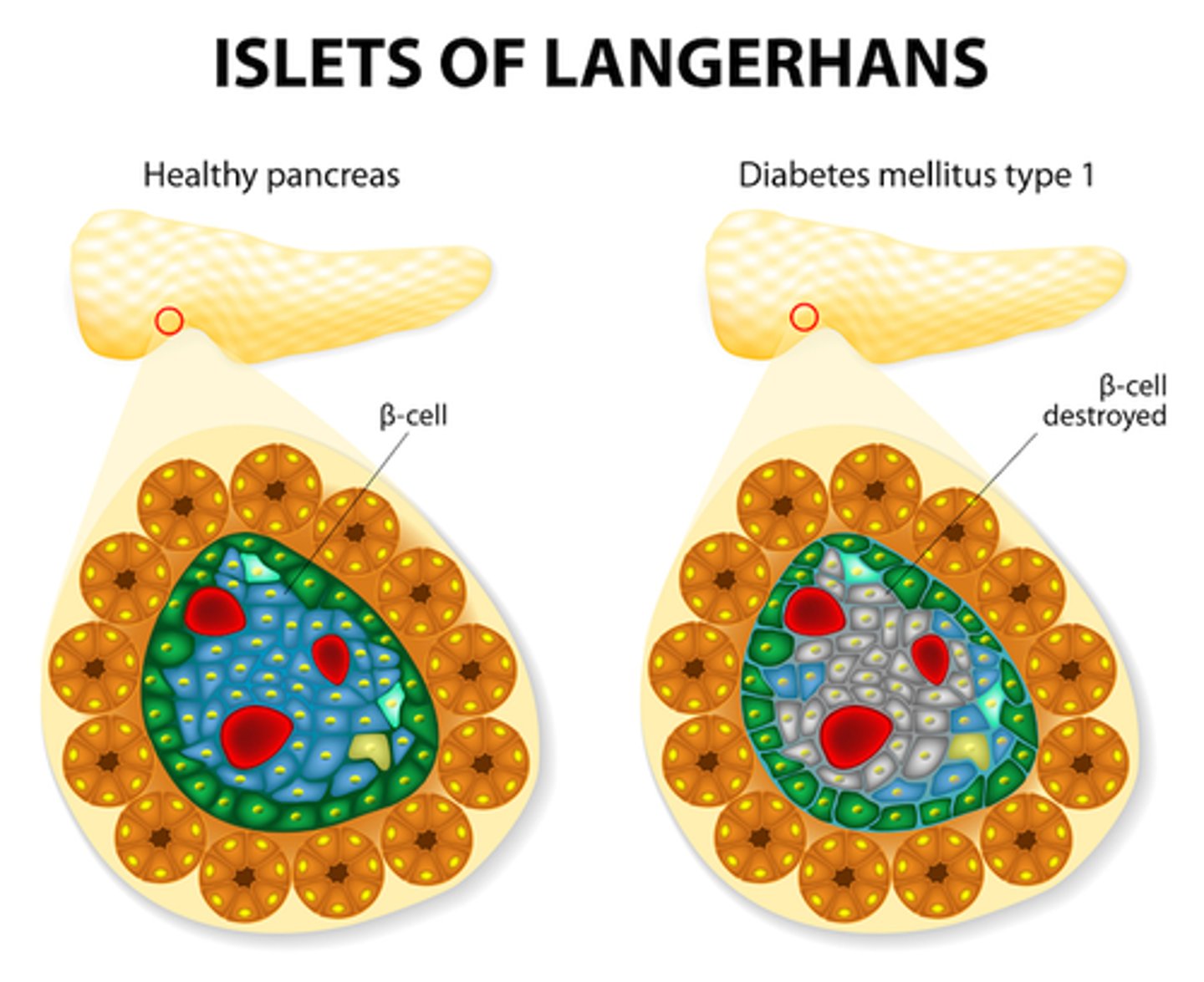
What is the pathophysiology of beta cell destruction?
- increased glucose in plasma (hyperglycemia) => high solute concentration (hypothalamus stimulates polydipsia/excessive thirst to try and dilute)
=> osmotic shift of fluid into circulation = cellular dehydration
- high solute concentration in renal tubules => Osmotic shift into filtrate = high urine production (polyuria stimulated)
- **Therefore: hyperglycemia, polydipsia, polyuria, glycosuria (hallmarks of DM I)
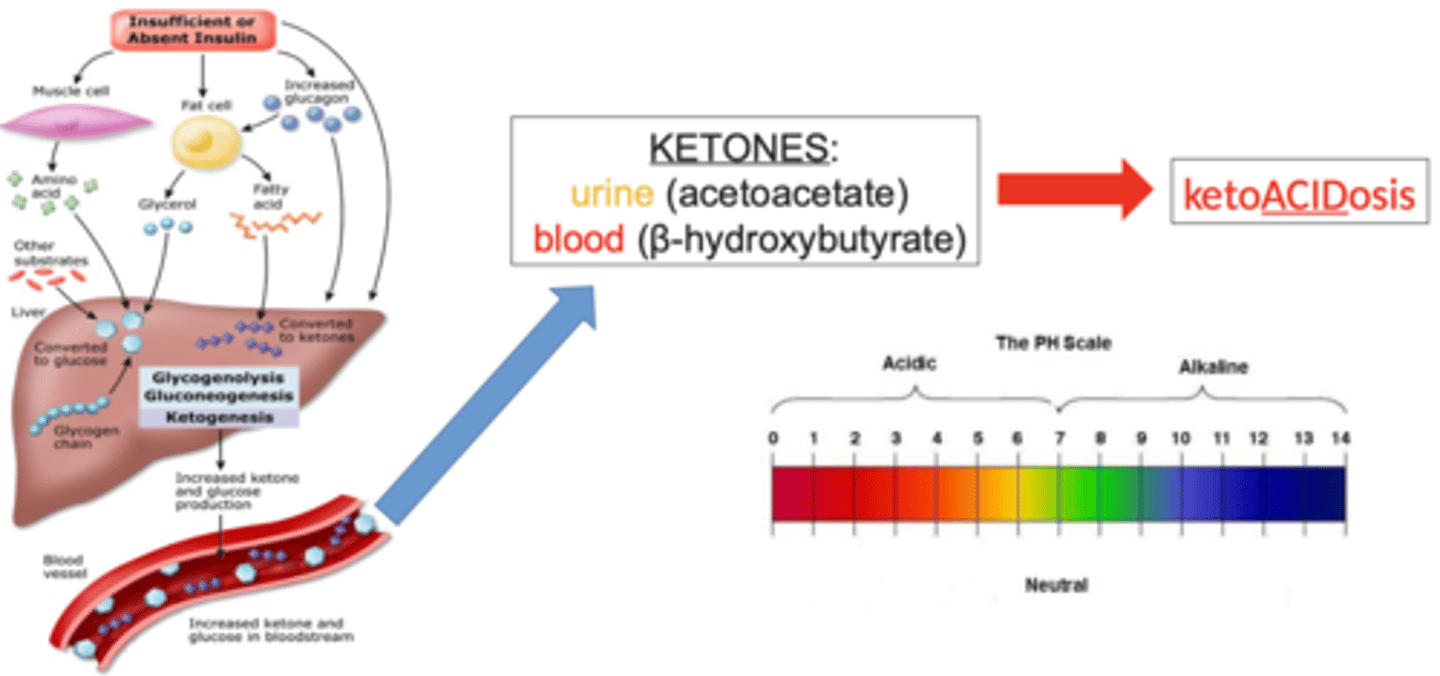
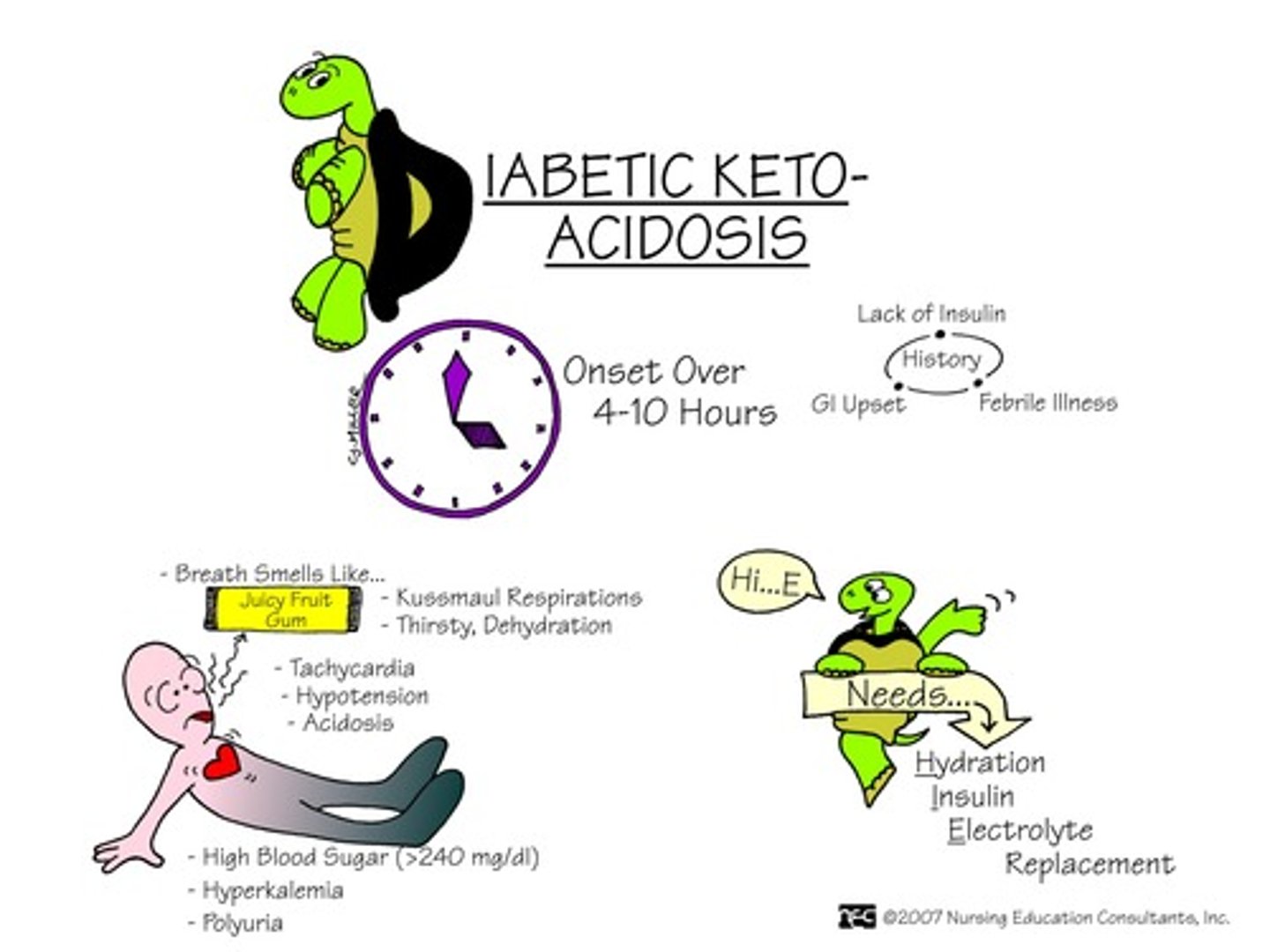
What happens with ketones due to beta cell destruction in DM I?
- metabolic shift to use fat for energy => breakdown of triglycerides & glycerol
- hepatic metabolism of fatty acids => ketone bodies produced
- ketones are acidic => causing metabolic acidosis
- & therefore: ketonuria, changes in LOC (due to metabolism alteration & metabolic acidosis), acetone breath (sweet), metabolic acidosis, coma, and maybe even death
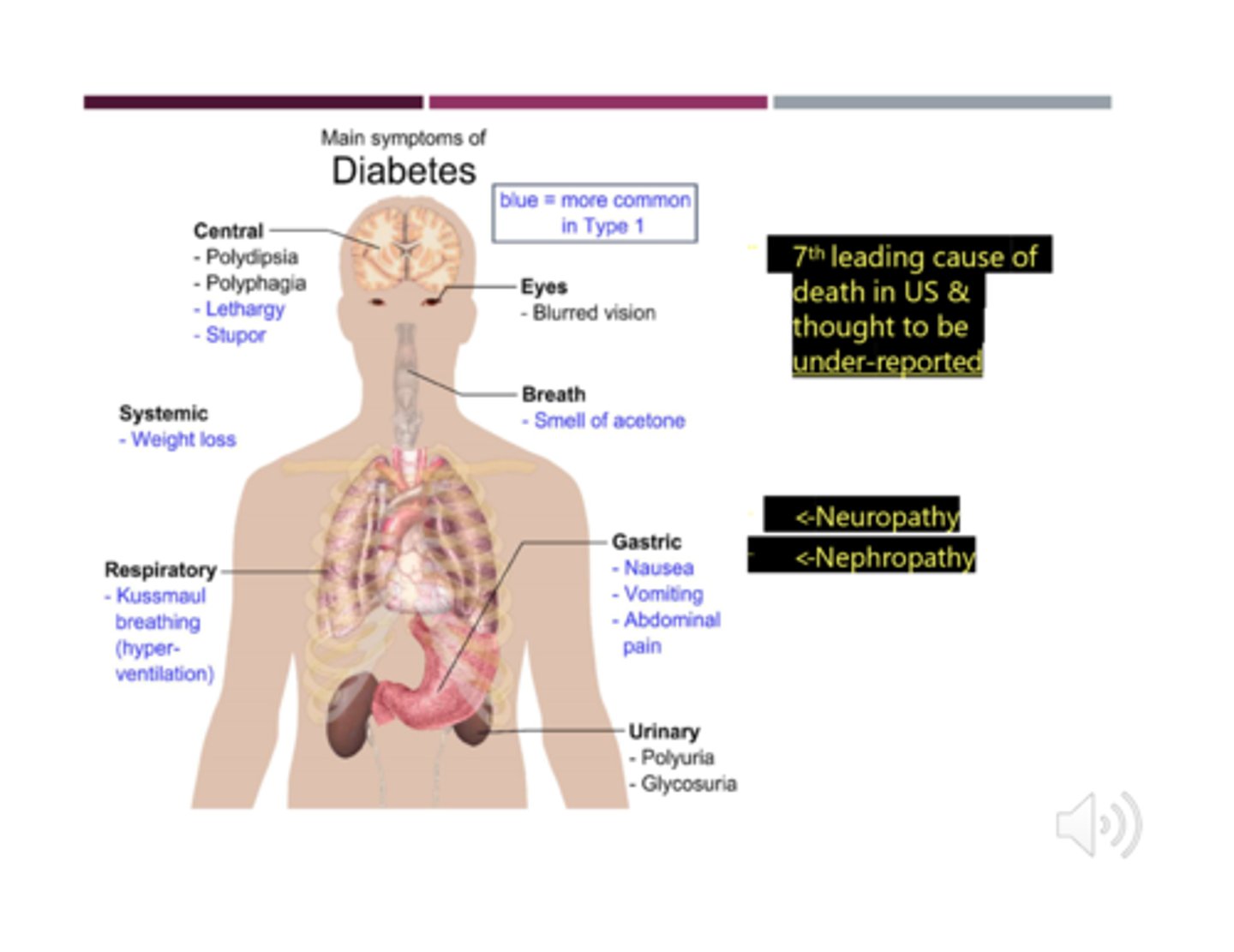
What is "acetone breath"?
- result of normal ketone metabolism, however if we are able to smell it this indicates ketones are present in high numbers (seen in DM I)
- commonly misunderstood as a patient being "too intoxicated" which is not the case! need to check.
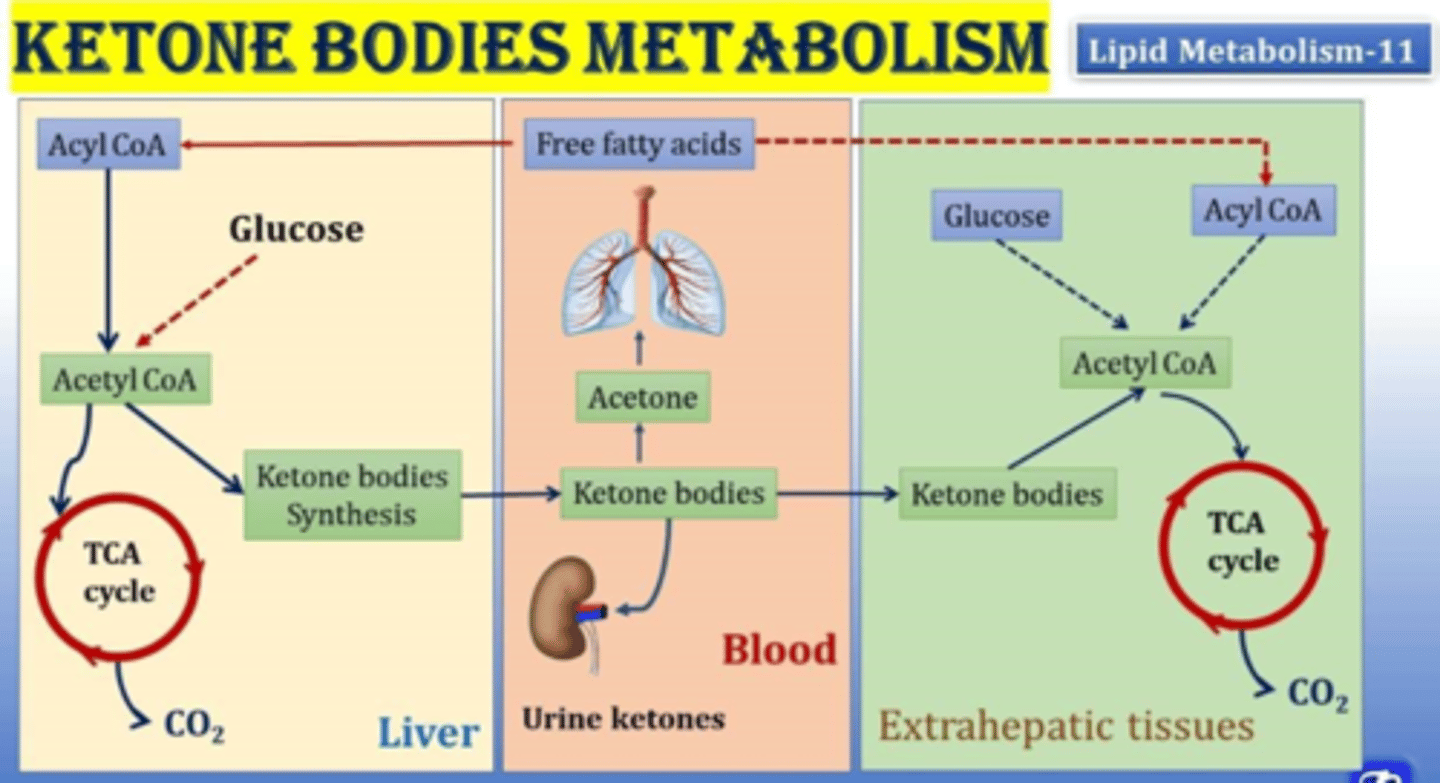
What happens in ketone body metabolism?
Free fatty acids => Acyl CoA => Acetyl CoA => Ketone bodies synthesis in liver => ketone bodies in blood => acetone => into lungs (sweet breath)
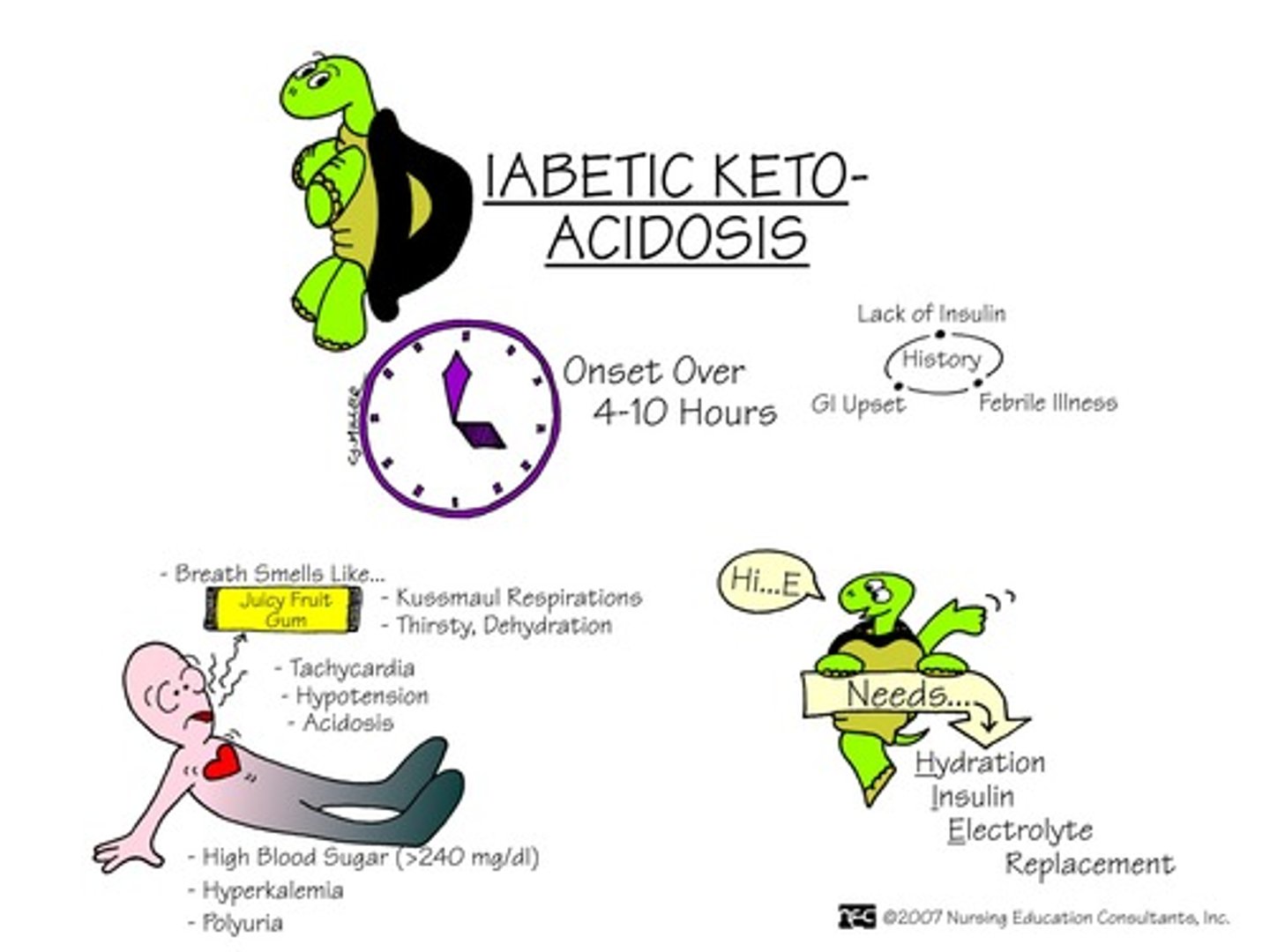
What is diabetic ketoacidosis (DKA)
When your blood turns acidic because there are too many ketones in your blood due to a lack of insulin
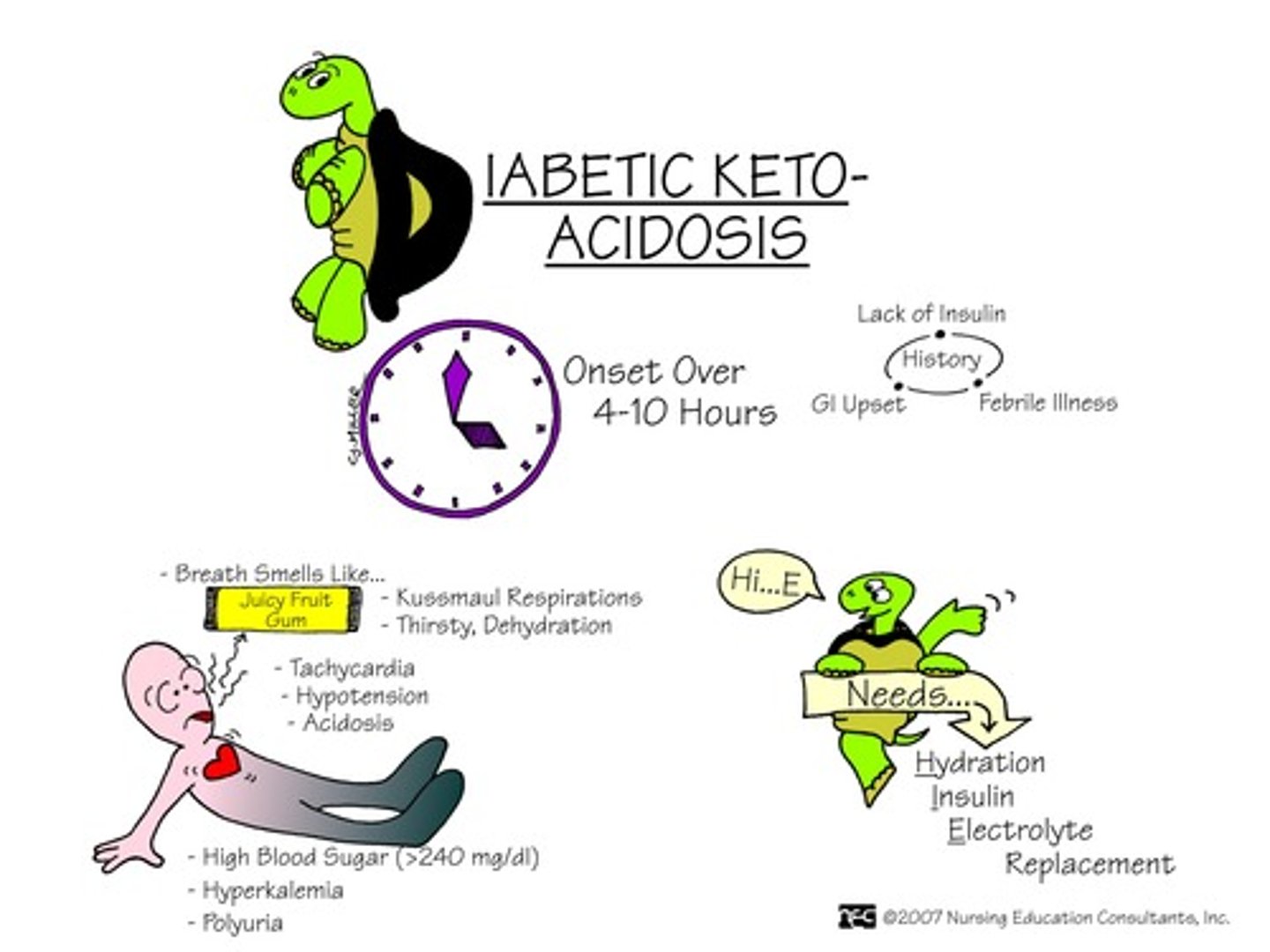
What are some energy substitutes for reduced glucose uptake?
- proteolysis => weight loss & muscle wasting
- lipolysis (fatty acid breakdown) => liver metabolism of fatty acids (fatty acid oxidation) = ketones & ketonuria
- causes altered cellular functions (eg. insulin resistance)
How does reduced glucose uptake in DM I cause injury to the body?
- injury: endothelial dysfunction + decreased angiogenesis (formation/repair of blood vessels) + increased oxidative stress =inflammatory consequences
- organ injury: retinopathy, neuropathy, nephropathy, CV
What to keep in mind when looking at ketone levels
trace amounts are fine but anything higher indicates impaired metabolism

What is diabetes mellitus type 1?
= total destruction of beta cells: IDDM (insulin dependent diabetes mellitus)
- dx: fasting glucose >7 mmol/L (normal <6)
- diagnosed: usually pre-30 yrs of age (toddlers; teens)
- 400 000 Canadians (out of 4 million who have DM1&2)
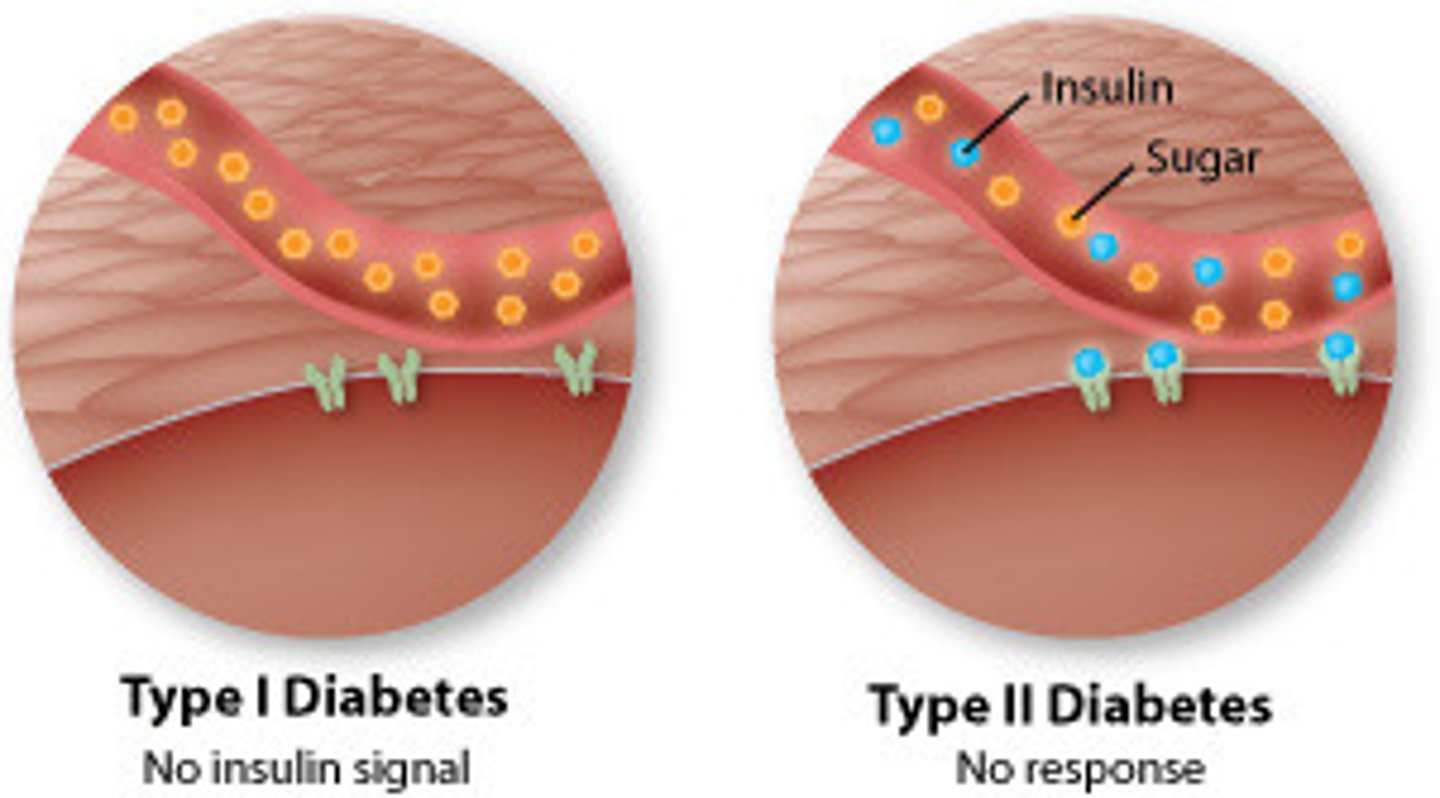
What is the difference between DM type 1a & type 1b?
- Type 1A: genetic predisposition + triggering event (eg. infection, trauma) (may or may not happen) => immune reaction to beta cell antigens = autoimmune (most common!!)
- Type 1B: idiopathic (nonspecific, familial), rare
How to tx DM & what happens if you don't tx?
- Tx: insulin (discovered by Banting/Best/McLeod; 1923 Nobel Prize) (both types)
- No Tx: diabetic ketoacidosis = death
What are endogenous insulin levels
- produced in pancreas
- insulin always measured in 'units' ("U" or "IU"
- basal level (long acting) = 5-15 IU/mL (clinically normal glucose level = 4-8 mmol/L => 4-8 feeling great)
- peak rise = 60-90 IU/mL
- tx therefore focuses on mimicking above
How is synthetic insulin formed?
Synthetically acquired from yeast formation in a petri dish

What is the goal of tx with insulin?
- restore normal glucose patterns (insulin administration will mimic basal & peak endogenous levels)
- minimize risk of hypoglycemia
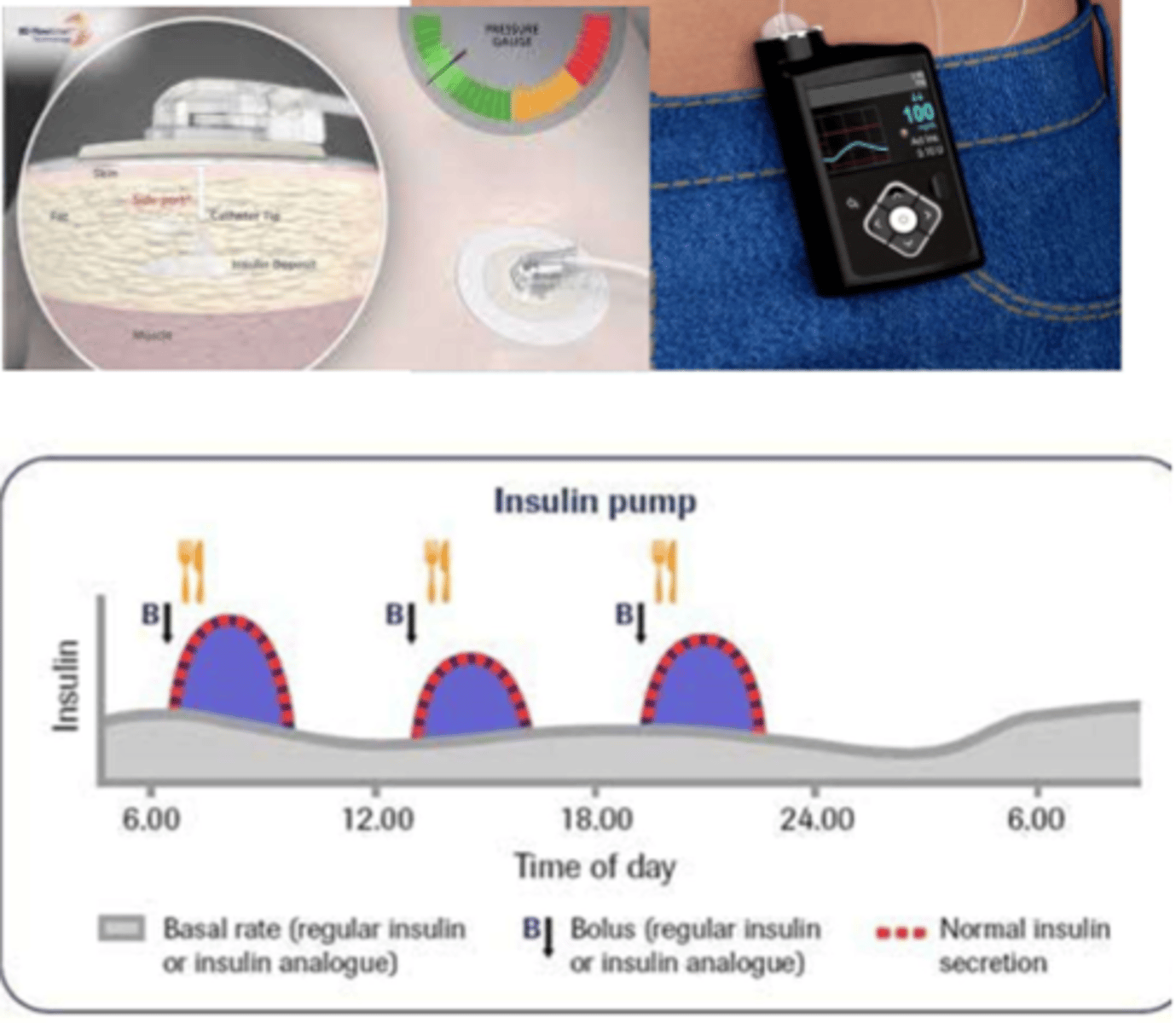
What are the differences between basal and bolus insulin
- basal: constant insulin, always available (long acting) (aka background replacement - supplies body when not eating)
- bolus: short acting insulin you get at mealtime (aka "mealtime bolus"), secreted from beta cells to meet requirements
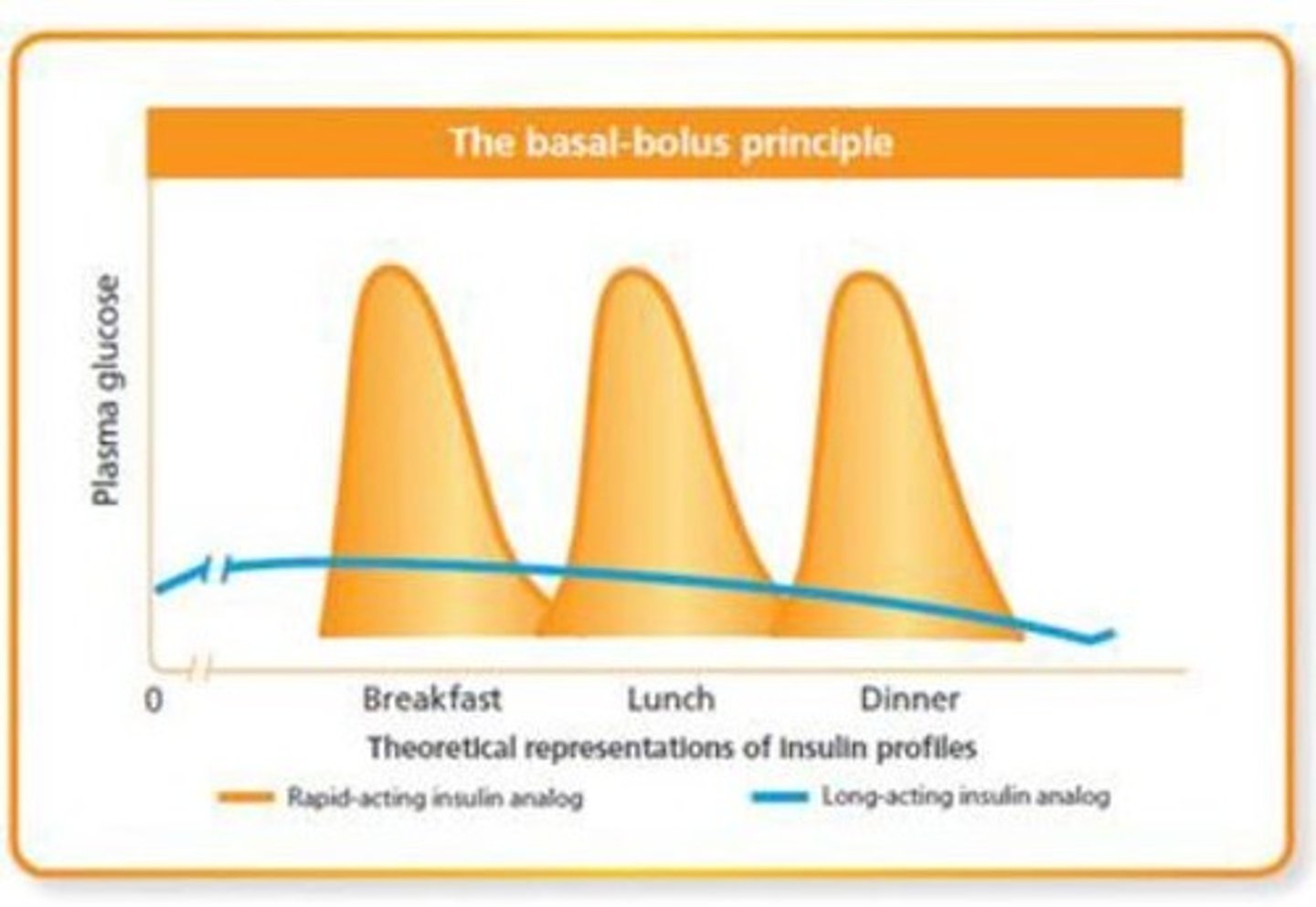
What are the 4 basic insulin preparation categories?
1) Rapid acting (mealtime bolus)
2) Long acting (basal, background)
3) Short acting ('regular' mealtime bolus)
4) Intermediate acting (basal, background)
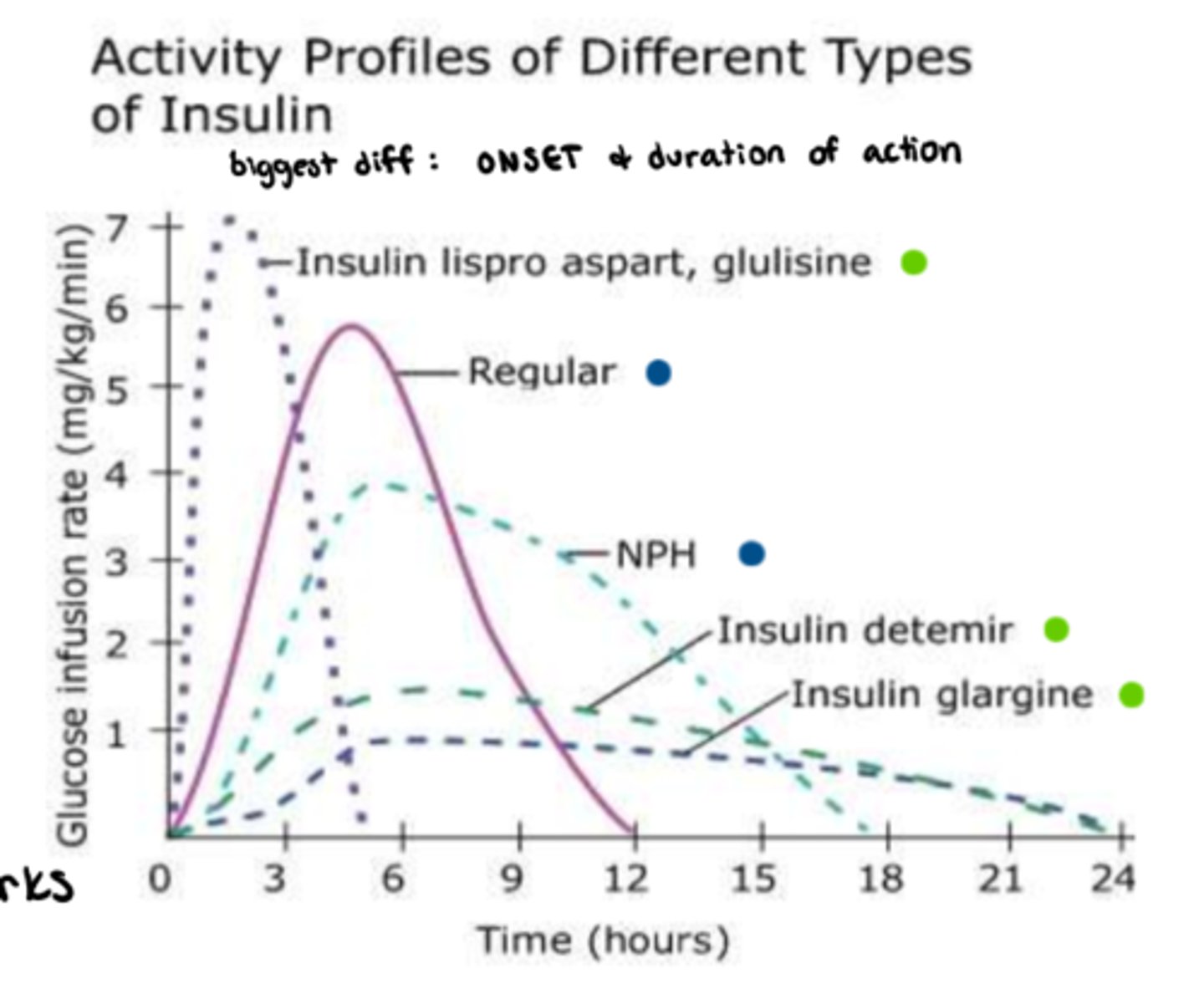
Which kinds of insulin is usually administered with rapid acting insulin?
long acting insulin

Which kind of insulin is usually administered with short acting?
intermediate acting insulin
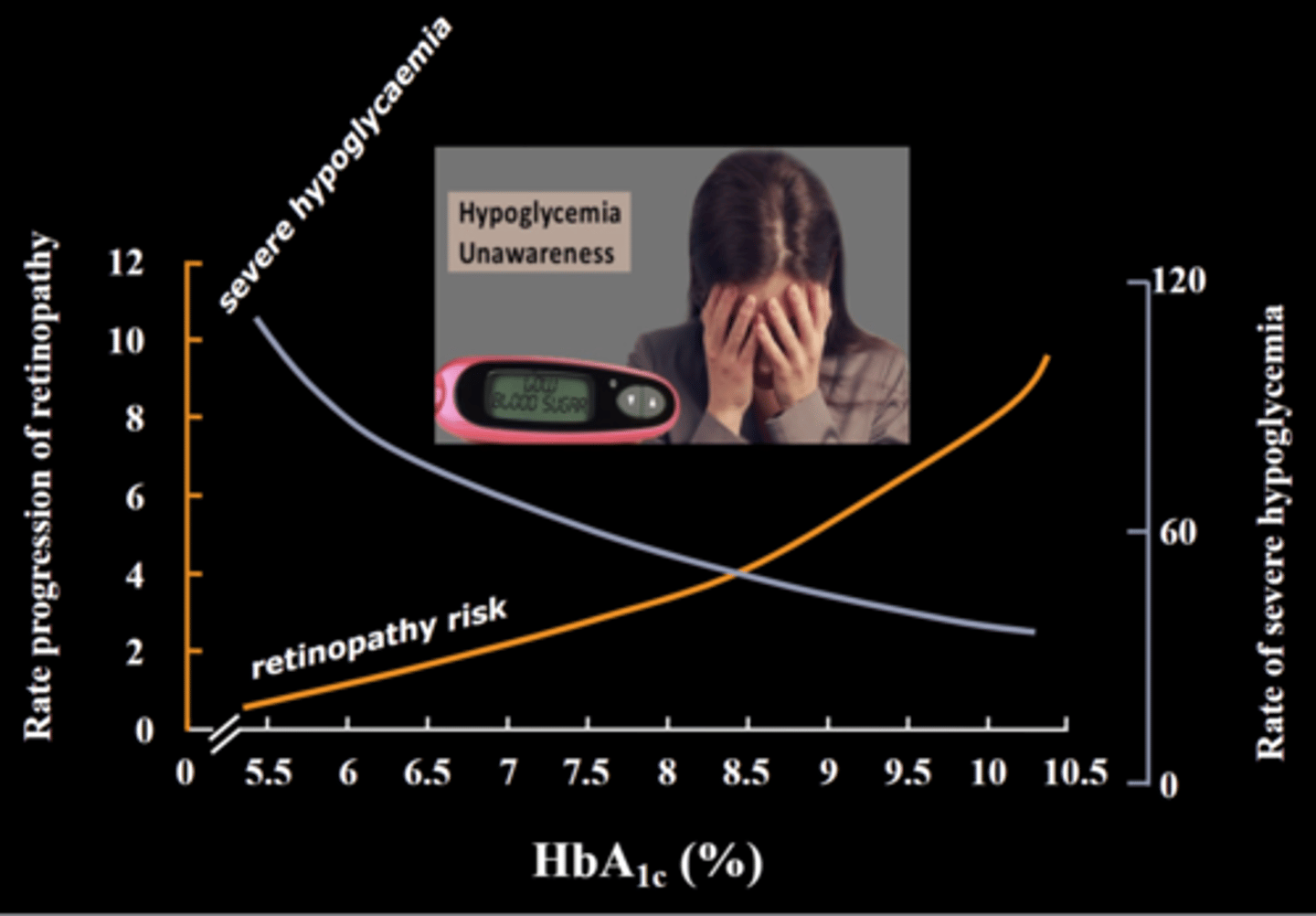
What is the biggest risk of hyperglycemia
Nighttime hyperglycemia => because you are unaware of S&S, causing harm long term

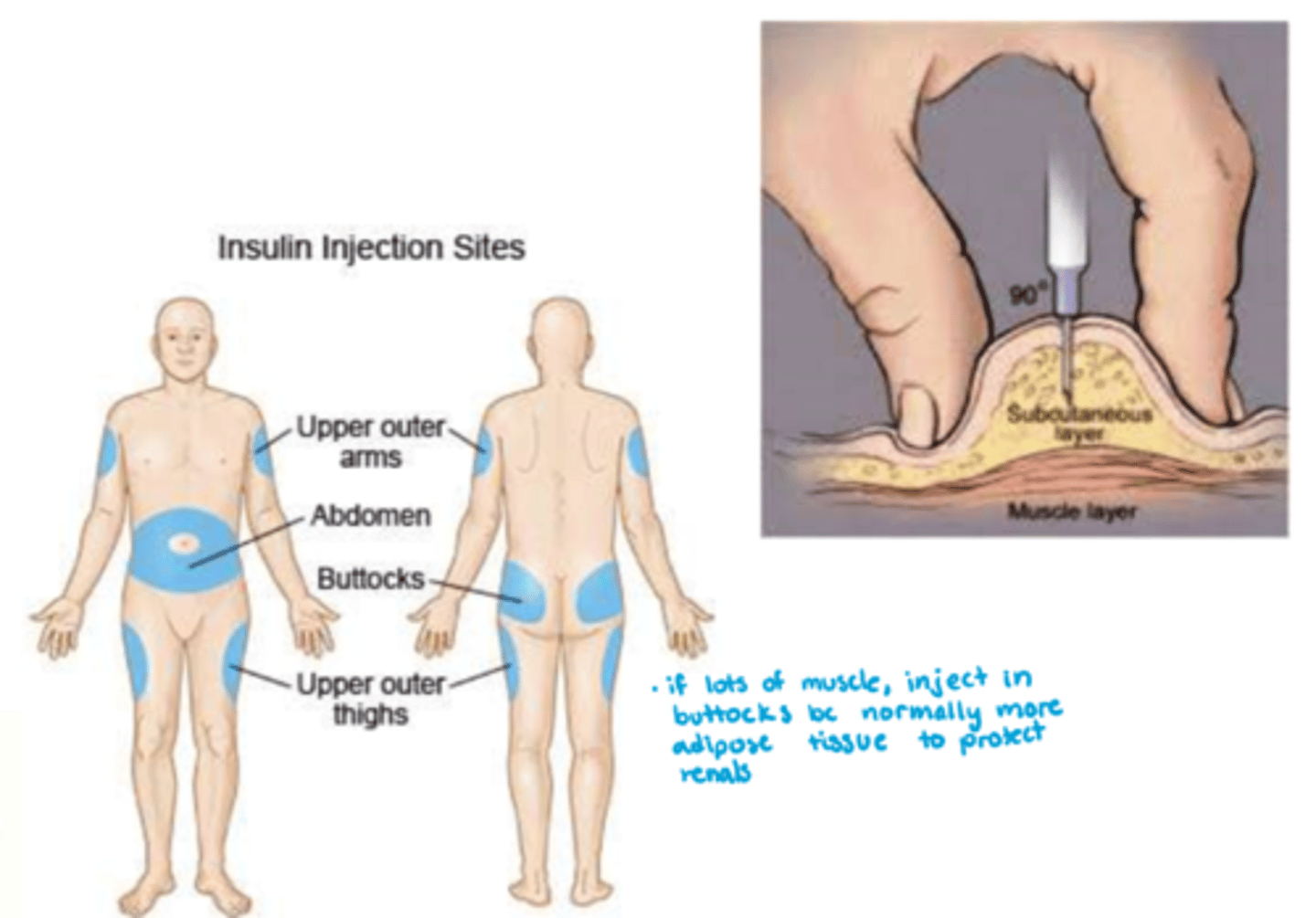
How is synthetic insulin administered?
- route: SC (note: IV utilized in critically ill patients) (want SC b/c muscle has too much perfusion)
- abdomen most commonly utilized unless you have a legit 8 pack!
- formulation: needle injections, portable pen injectors, insulin pumps - basal & bolus delivery
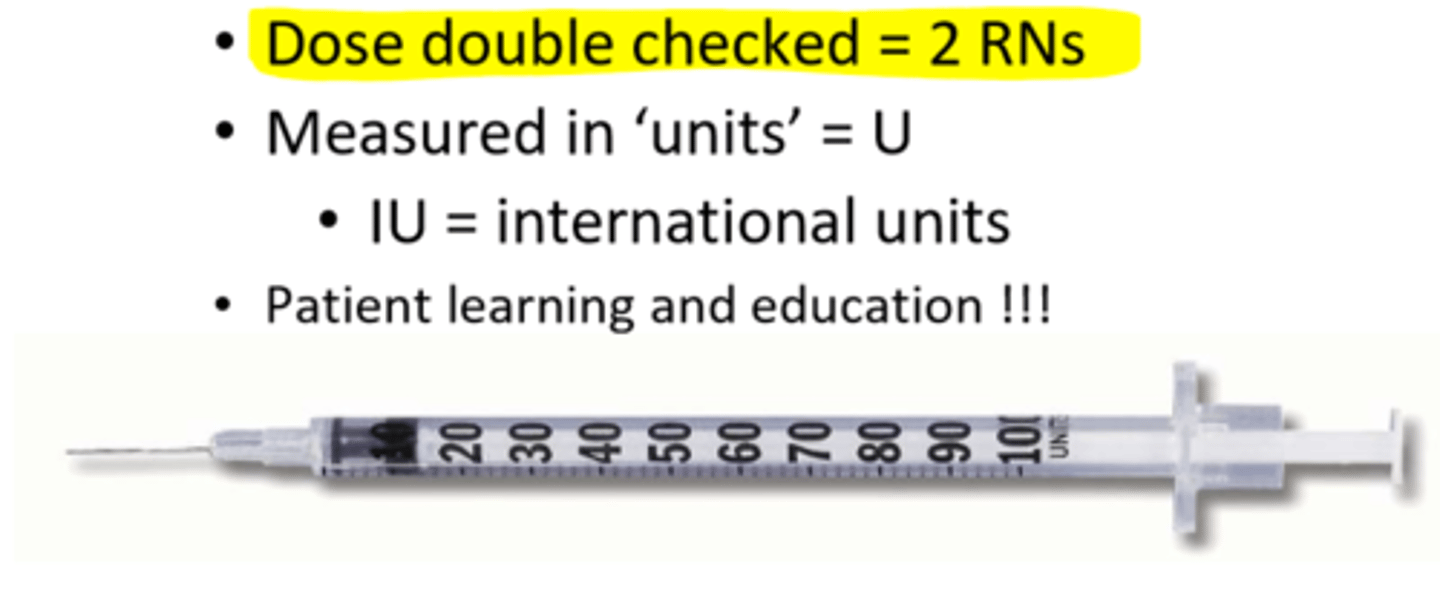
How to ensure 7 rights are checked for insulin admin?
- dose double checked = 2 RNs
- always rotate sites to prevent tissue injury & decrease in fx
- measured in 'units' = U (IU = international units)
- patient learning and education !!!
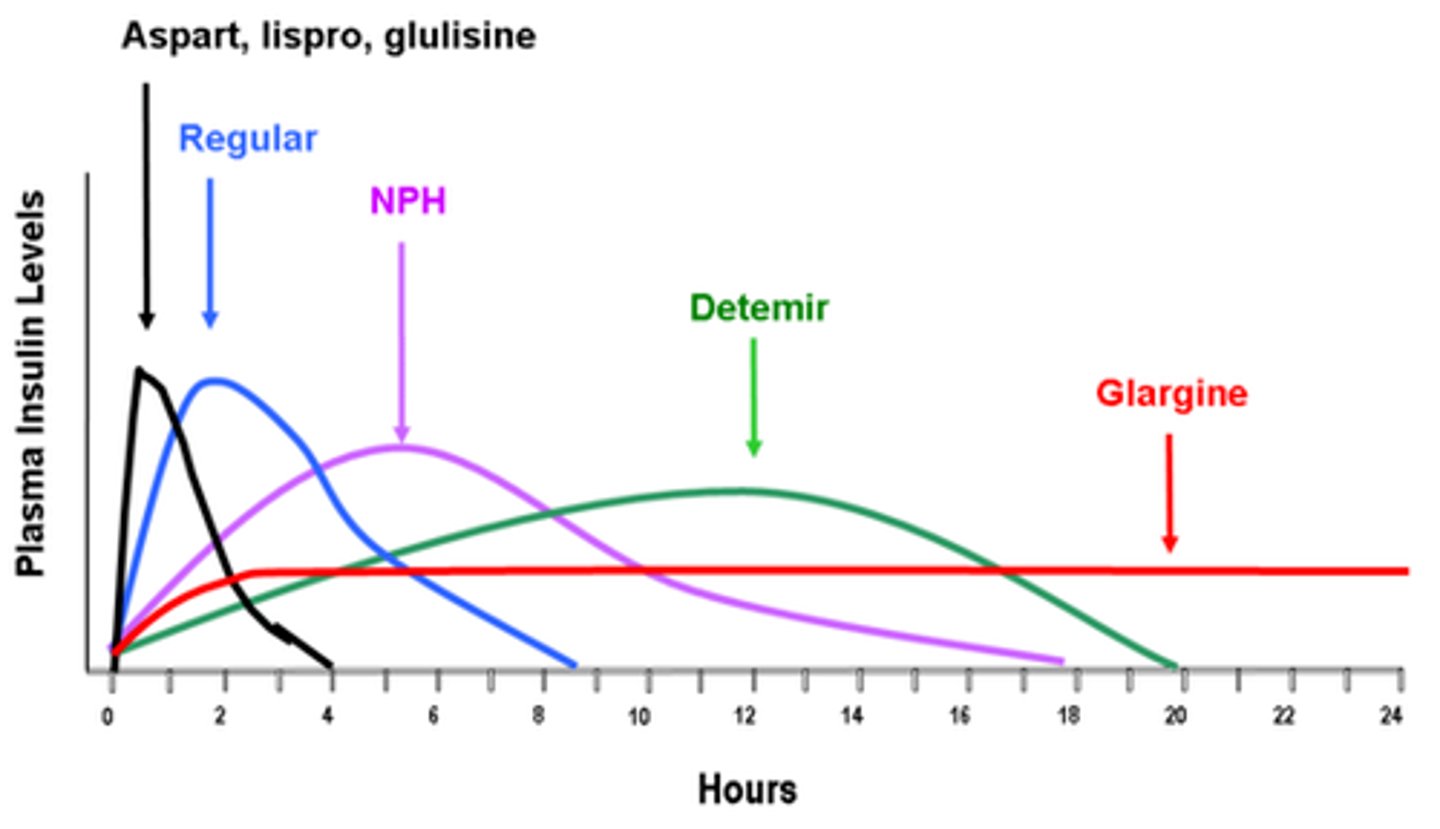
What is Rapid acting Insulin
- onset: 10-15 minutes
- peak: 1-2 hrs
- duration: 3-5 hrs
- ideal for: meal-time bolus, pt eats right away! (food tray must be ready!!!)
- good in insulin pumps, not IV
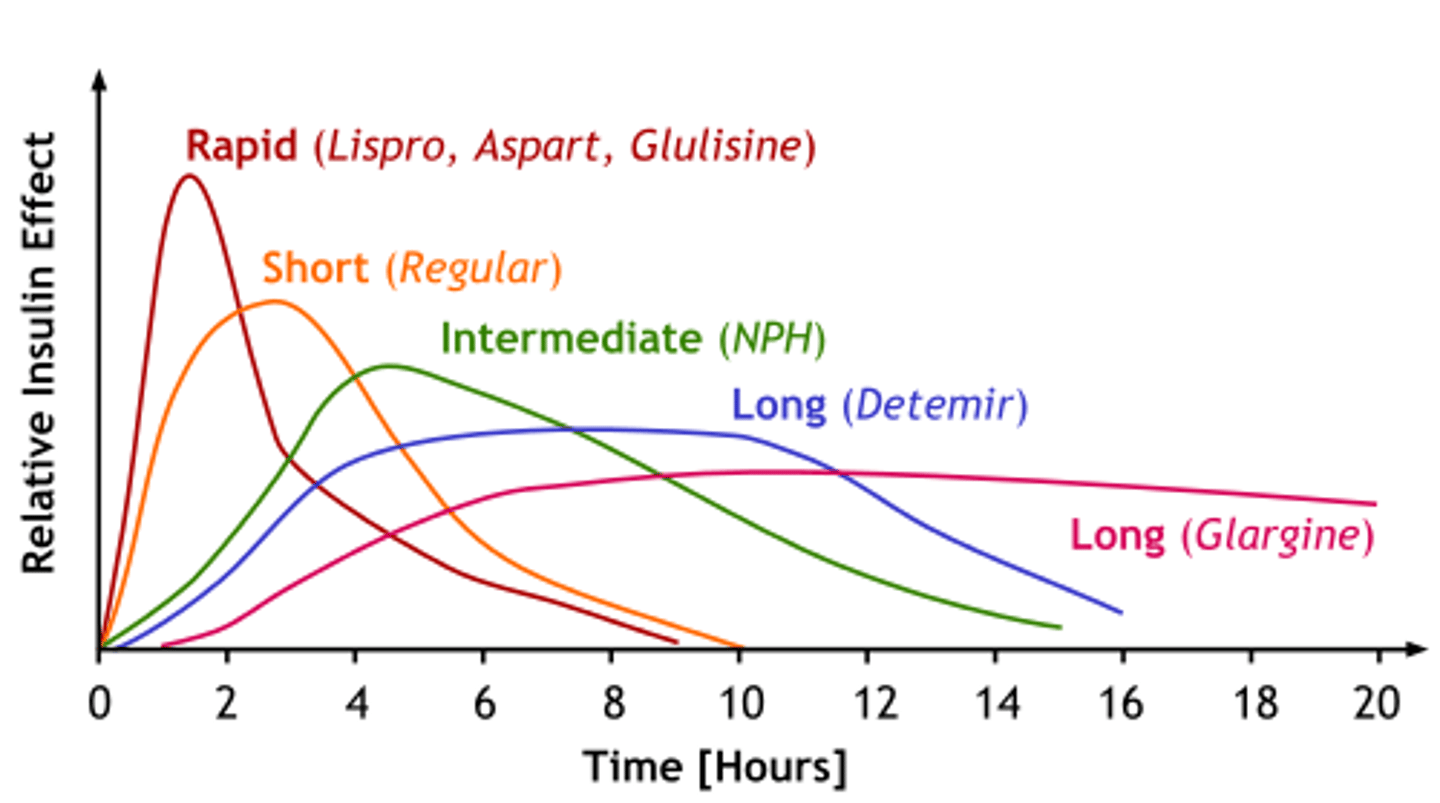
What are the rapid acting insulin drugs of choice?
- Humalog (Lispro)
- Novorapid (Aspart)
- Apidra (Glulisine)
- Fiasp (Aspart) => onset: 4 min

What to note about Fiasp admin in rapid acting insulin?
improved mealtime control, administer immediately prior or during meal
What is 'rapid acting' insulin in pumps like in meal time boluses?
- per carbohydrate content
- glucose level pre-meal
- check: glucose levels 1-2 hr post
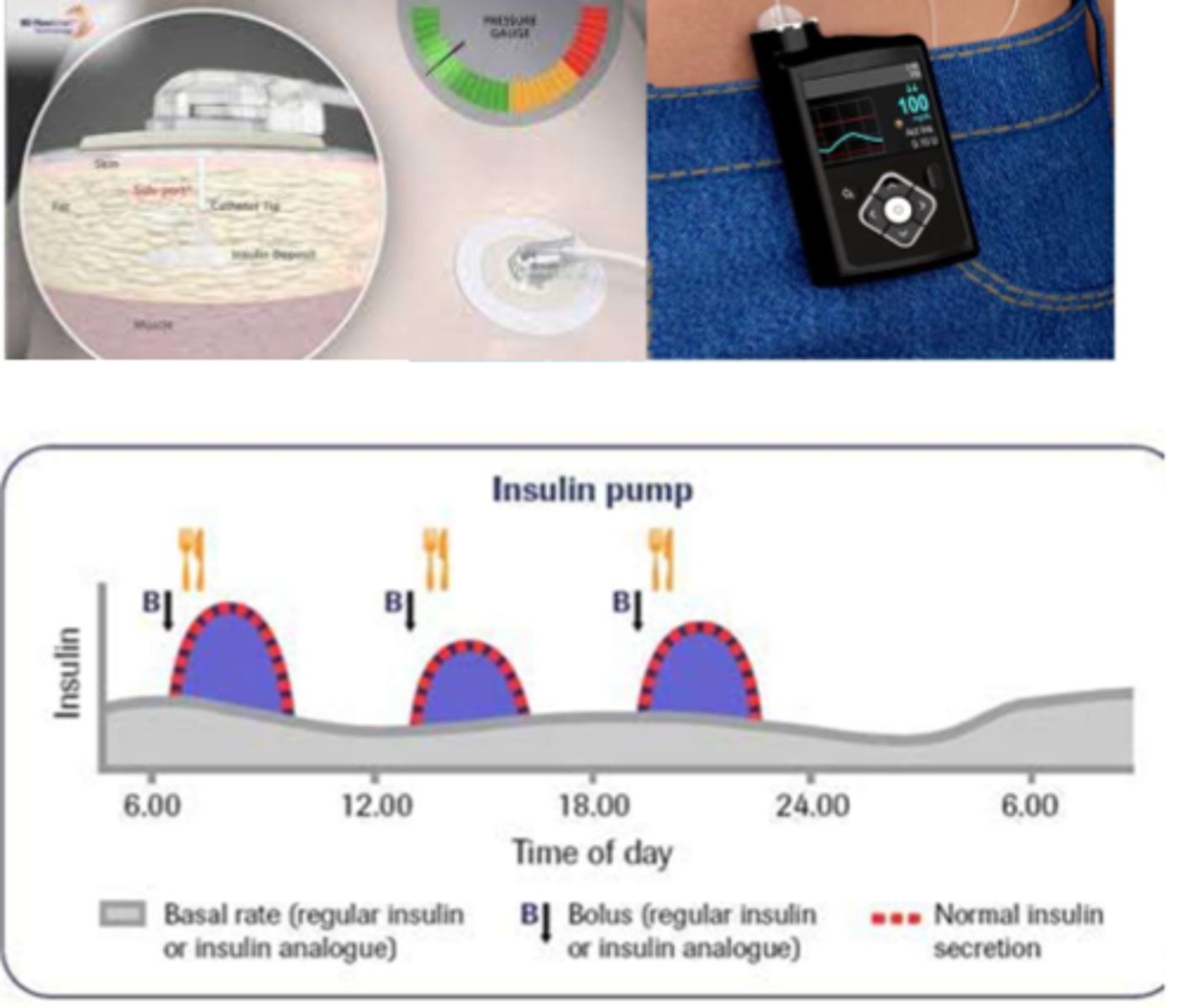
What is 'rapid acting' insulin pumps like in basal insulin?
- slow infusion of rapid acting insulin over 24 hrs (ongoing trickle)
- careful monitoring (bedtime BG)
- endocrinology appointments
What is long acting Insulin?
- Onset: 90 min
- Plateaus for: up to 24 hrs
- ideal for: background, admin. 1-2x daily (consistency is important), never IV
What are the long acting insulin drugs of choice? (hint: L is for long acting, except when it's Tresiba)
- Levemir (Detemir)
- Lantus (Glargine)
- Tresiba (Degludec) => ultra-long acting (>30 hrs)
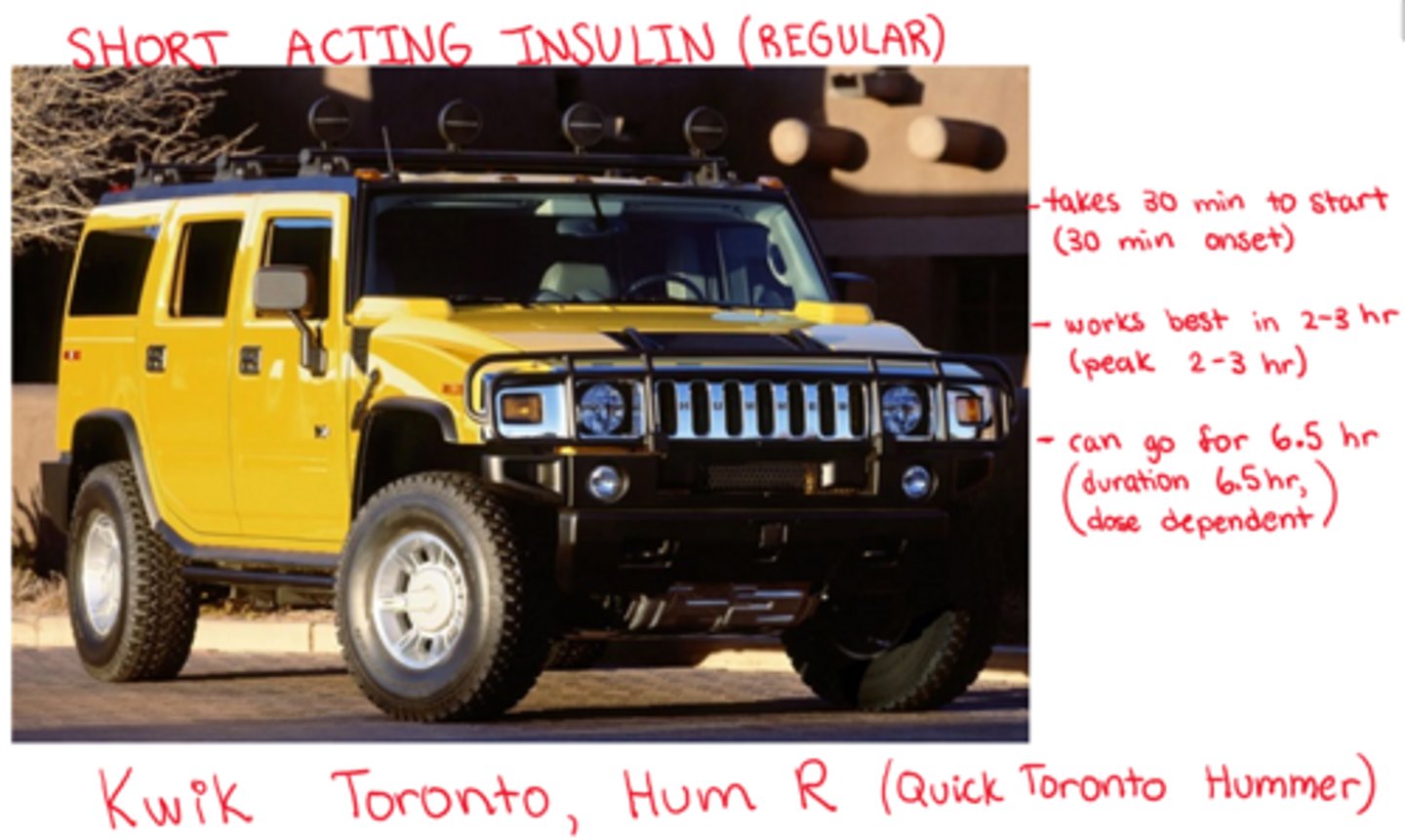
What is short acting Insulin ('regular')
- onset: 30 minutes
- peak: 2-3 hr
- duration: 6.5 hrs (dose dependent!)
What is short acting insulin ideal for?
- ideal for: meals (must be 30-45 min pre-meal!) BUT issues
with hypoglycemia & balancing dose w intake; pt eats!!!
- used IV if ketoacidosis or a new dx

What are the short acting insulin drugs of choice?
- Novolin ge Toronto
- Humulin R
- Entuzity (KwikPen) => 5x more concentrated; for high daily insulin requirements
What is intermediate acting Insulin?
- Onset: 1-3 hrs
- Peak: 5-8 hrs
- Duration: up to 18 hrs (dose dependent!)
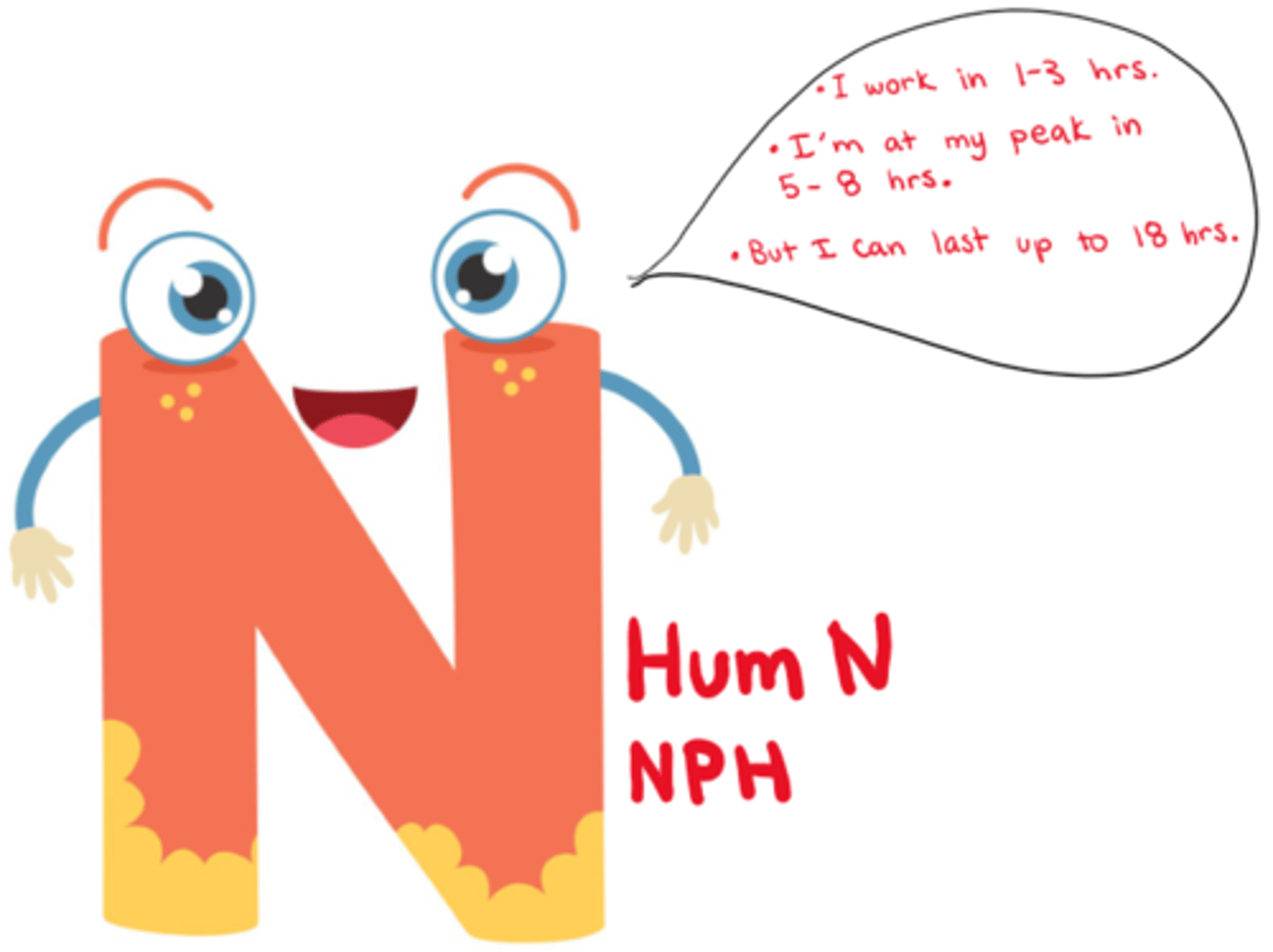
What is intermediate acting insulin ideal for?
- ideal for: background replacement, admin. 1-2x daily
- if pt on steroids (can match sugar peaks; monitor for night hypoglycemia!!! (evening snack important)
- never IV

What are the intermediate acting insulin drugs of choice?
- Humulin N
- Novolin ge NPH

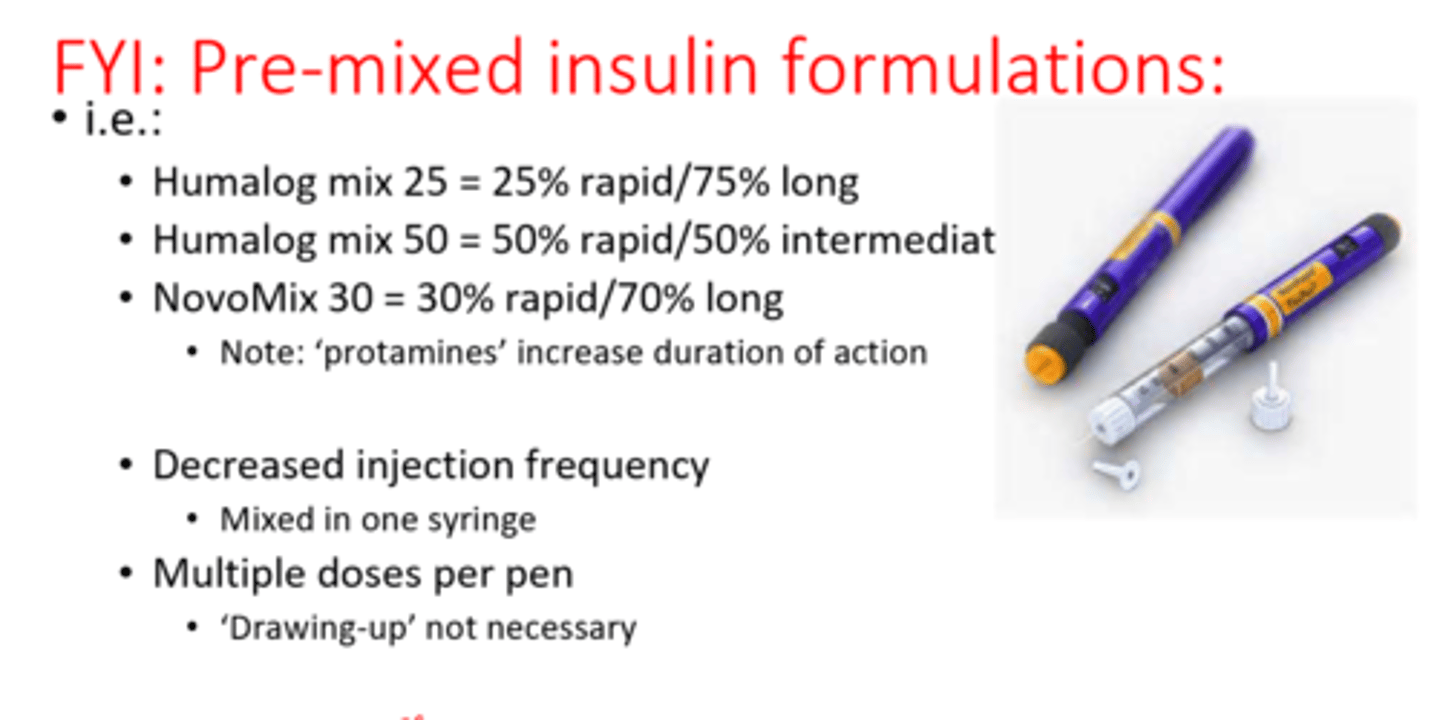
What is there to note about pre-mixed insulin formulations?
- decreased injection frequency
- mixed in one syringe
- multiple doses per pen, 'drawing-up' not necessary
- if a pt brings this in on their own, we would not use it on them. We would admin something else
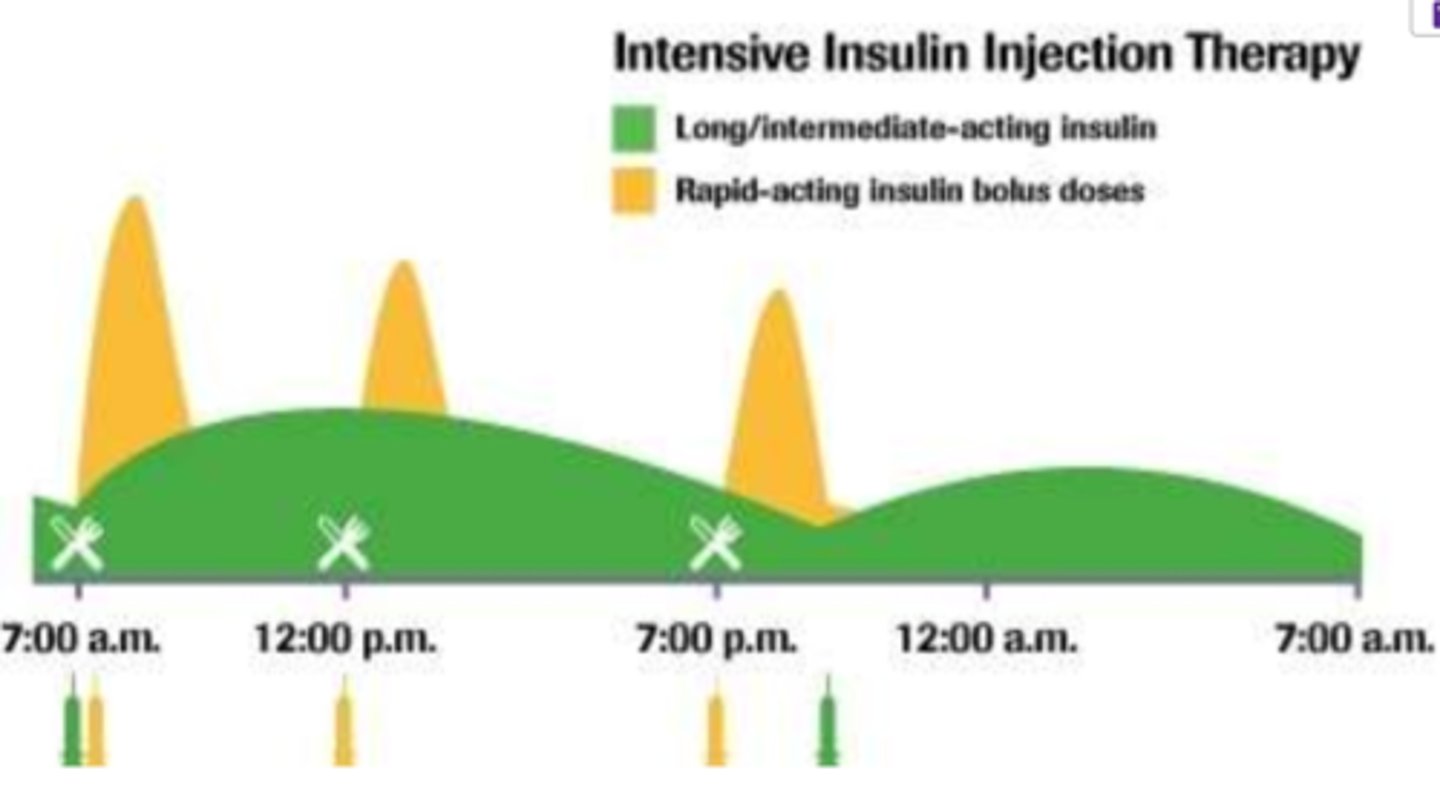
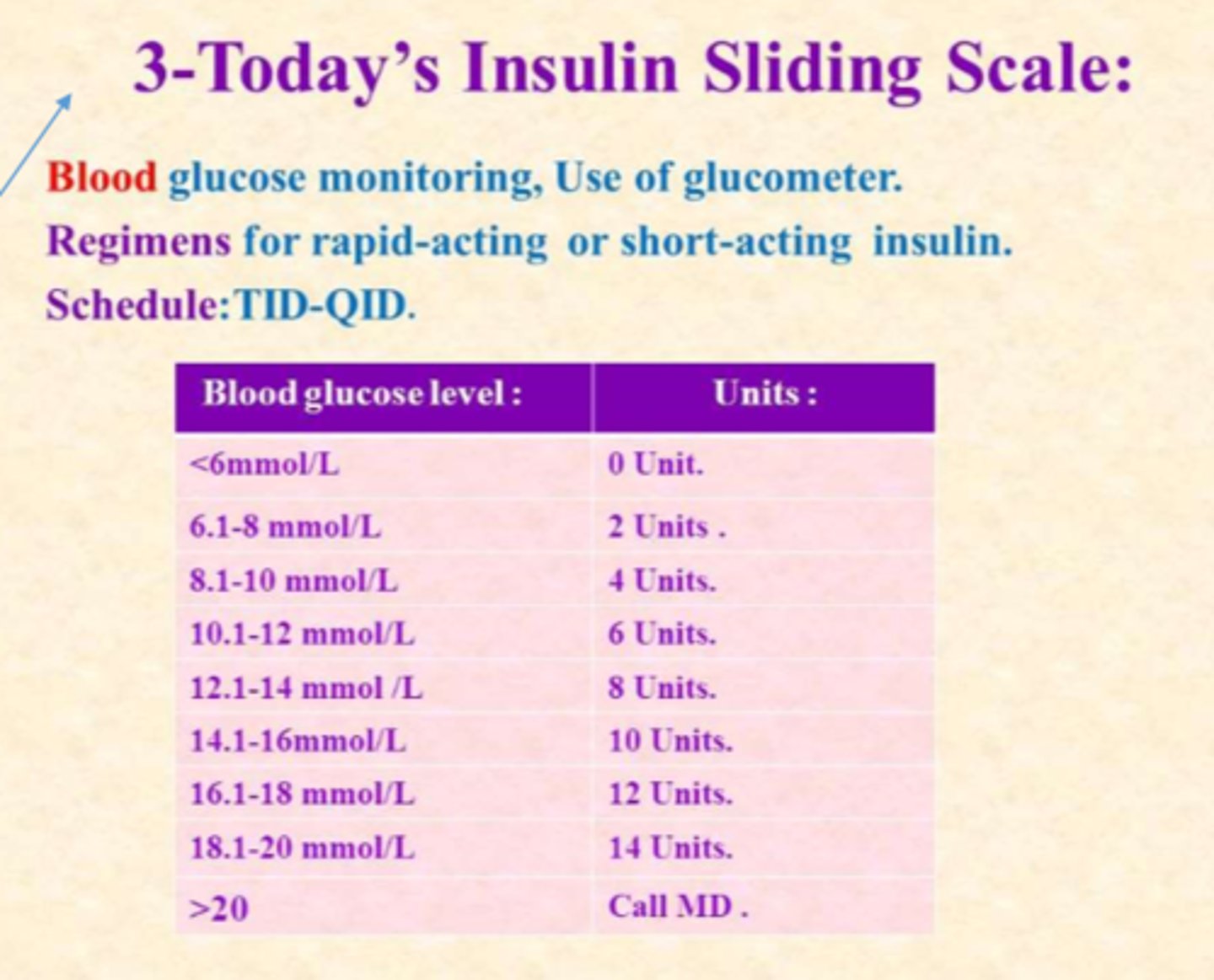
What is the estimated guide for intensive insulin tx requirements?
- for DM I patients
- estimated daily insulin requirement (U) = 0.55 (U) x Pt. wt (kg)
*note: estimate does not factor in BMR, activity/stress, food => it is a starting point
- approx. 40% of above = background requirement (basal)
- other 60% = meal-time (boluses)
What info is required for actual specific dosing of insulin
- plasma (blood) glucose (BG)
- carbohydrate content/meal (diabetic diet)
- goal: normal plasma glucose
What is BBIT?
- Daily routine serum glucose monitoring & insulin administration
- works to mimic normal daily insulin by using different forms of insulin admin together

What does 'BBIT' stand for?
- B = basal (long acting Insulin) in am
- B = bolus (short/rapid acting Insulin) at meals
- I = Insulin correction if necessary (short/rapid acting) (based on BG)
- T = titrate doses to achieve glucose levels 4-8 mmol/L (monitor glucose)
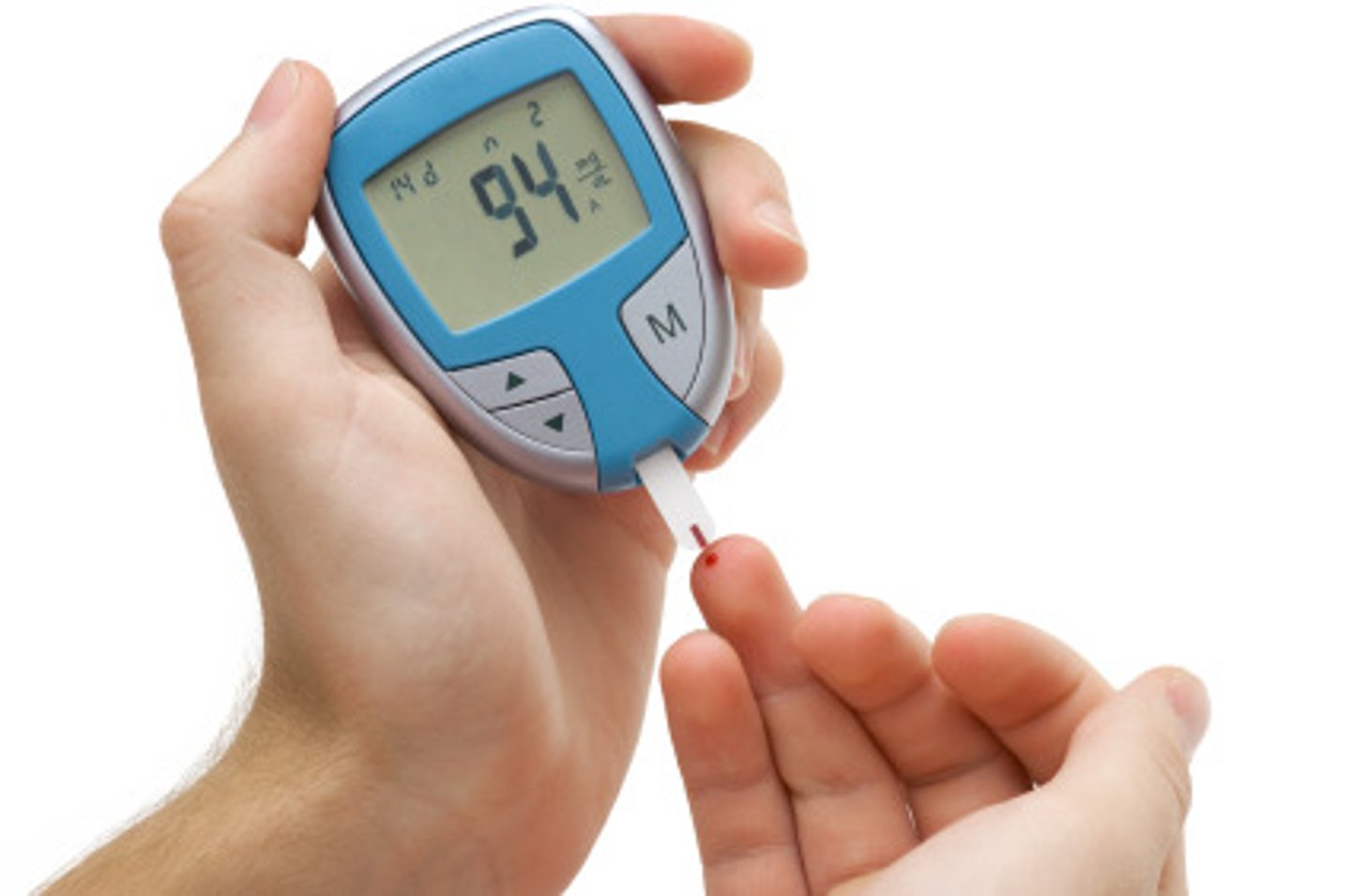
What are the recommended blood glucose checks?
- PRE-each MEAL
- POST-MEALS (if new dx)
- at BEDTIME
(4x per day is minimum requirement, 8 for newly diagnosed patients => pre+post meals; bedtime; nighttime)
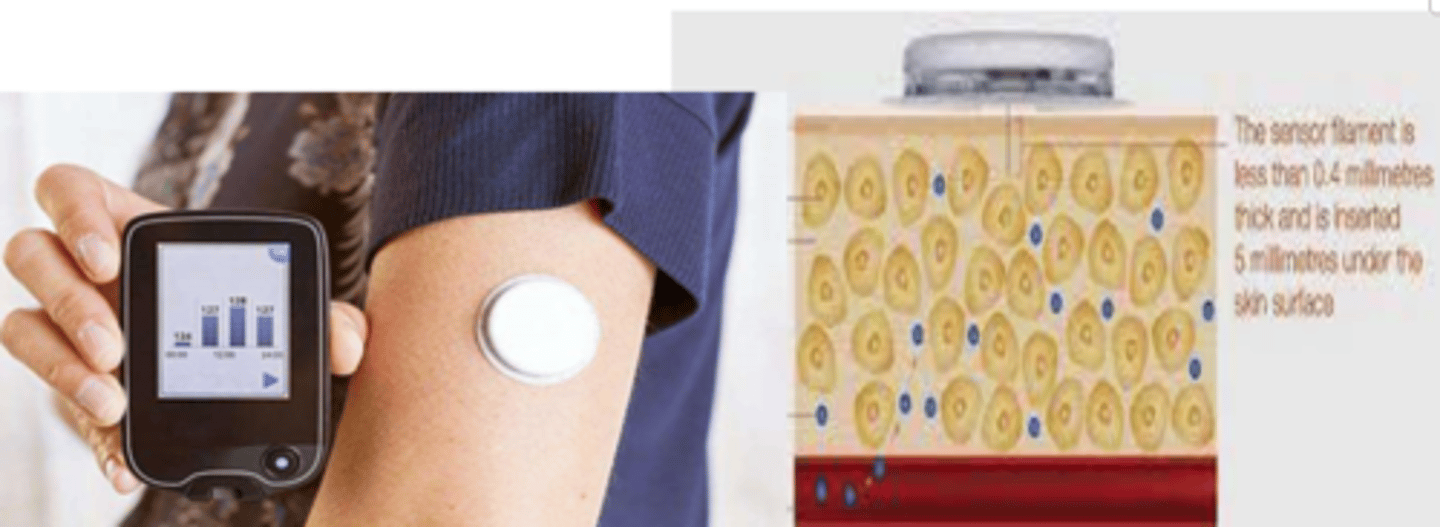
What different ways can we check blood glucose levels?
- glucometer: checks blood glucose via lancet (prick) of blood
- indwelling glucose sensory monitor: constantly measures
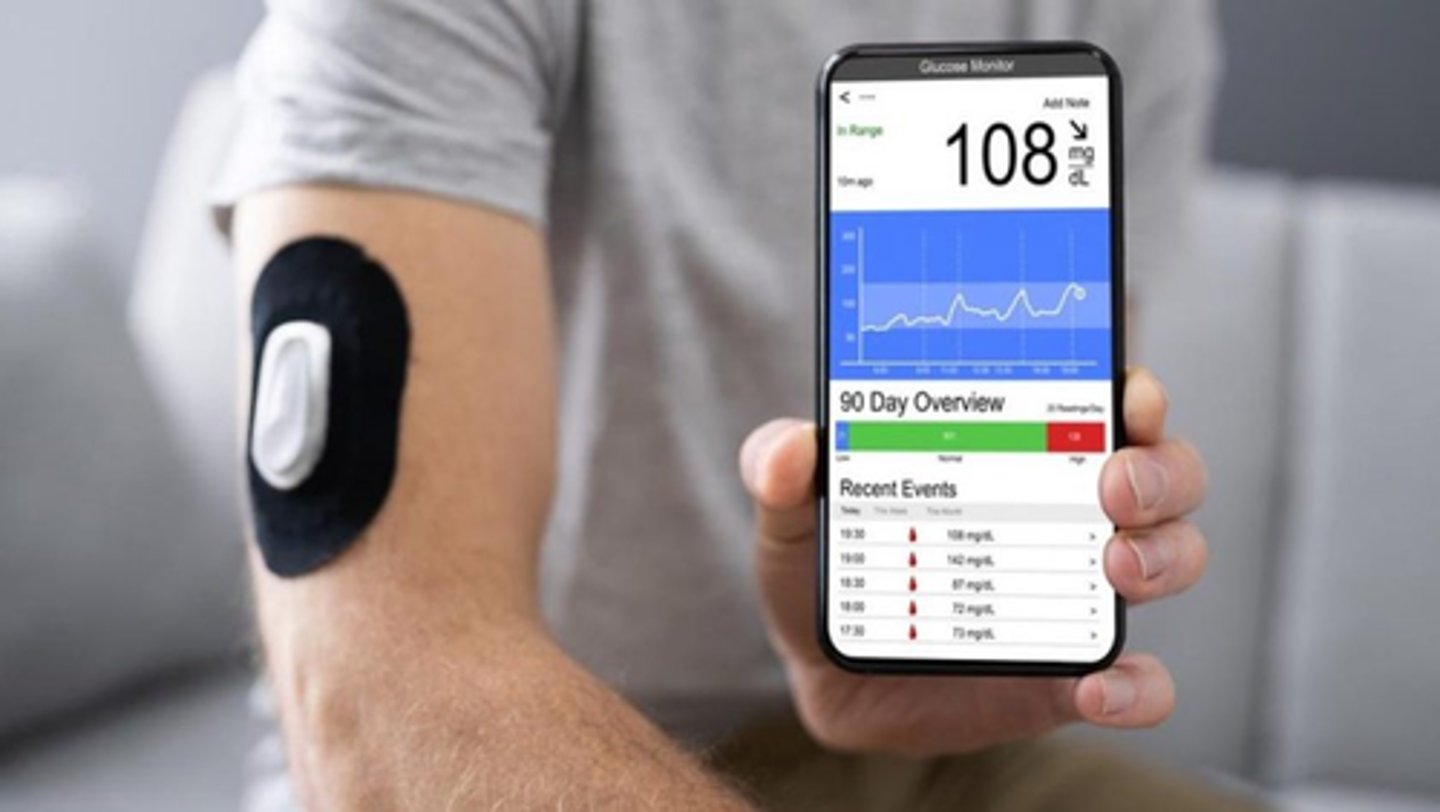
What is the BG to unit conversion
2.5 BG = 1 unit
What is BASAL insulin?
- long acting insulin, usually administered in a.m.
- similar amount each day, as long as the pre-dinner sugar is 'normal'
- preferably given in a.m. to avoid night hypoglycemia
- tweak amount based on blood sugar at 12 hr mark (bedtime BG reading)
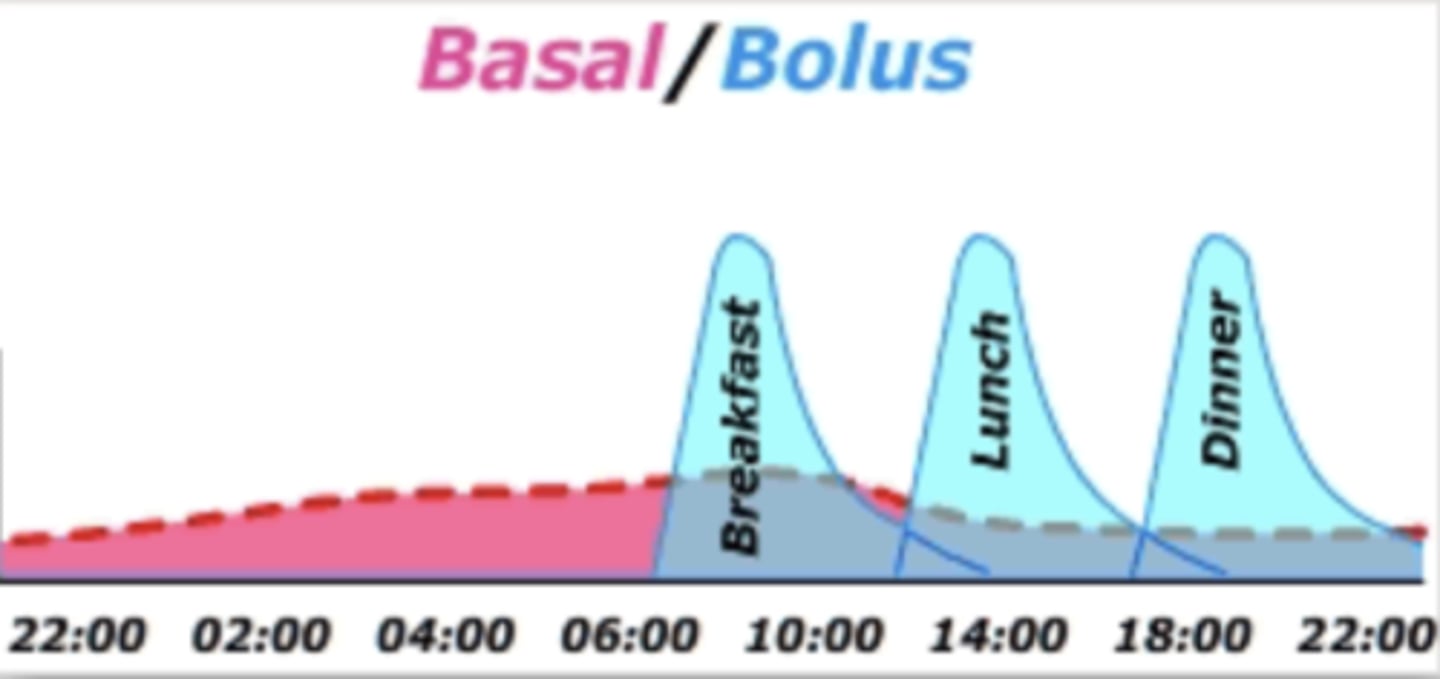
What is a continuous infusion of basal insulin?
IF patient on insulin pump, the basal dose is continuously delivered in a fast acting insulin form => therefore continuous infusion
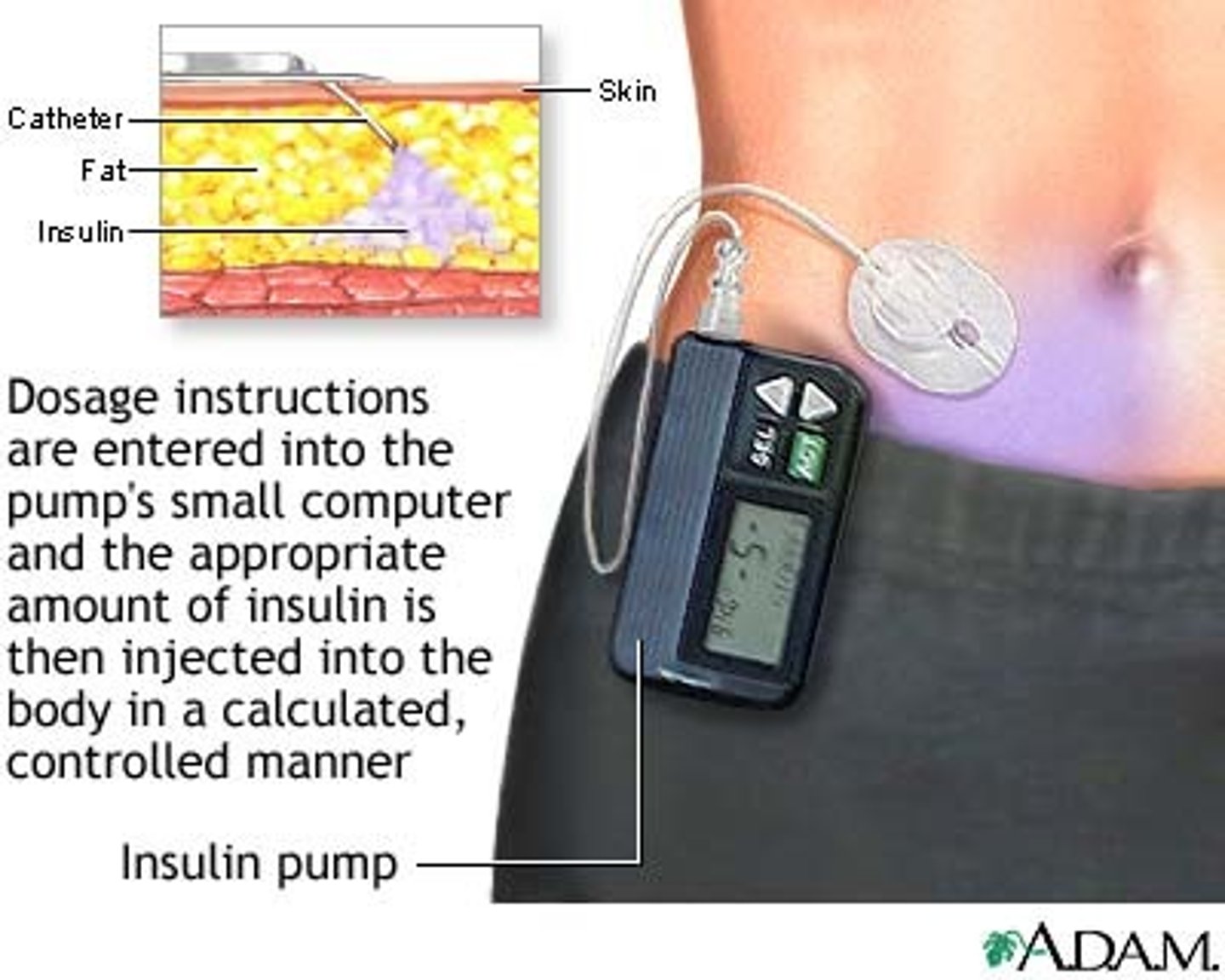
What matters a lot for accurate bolus dosing of insulin?
DIET!!

How to measure carbohydrate intake? (for bolus dosing)
- 'Carb counting' (45-60 g/meal)
- Carb total - fibre = total count (what insulin is based on)
- 15g of carbohydrate = 1 unit rapid acting insulin (on average)

What is the goal of a diabetic diet?
Goal: reduce hypo/hyperglycemia swings
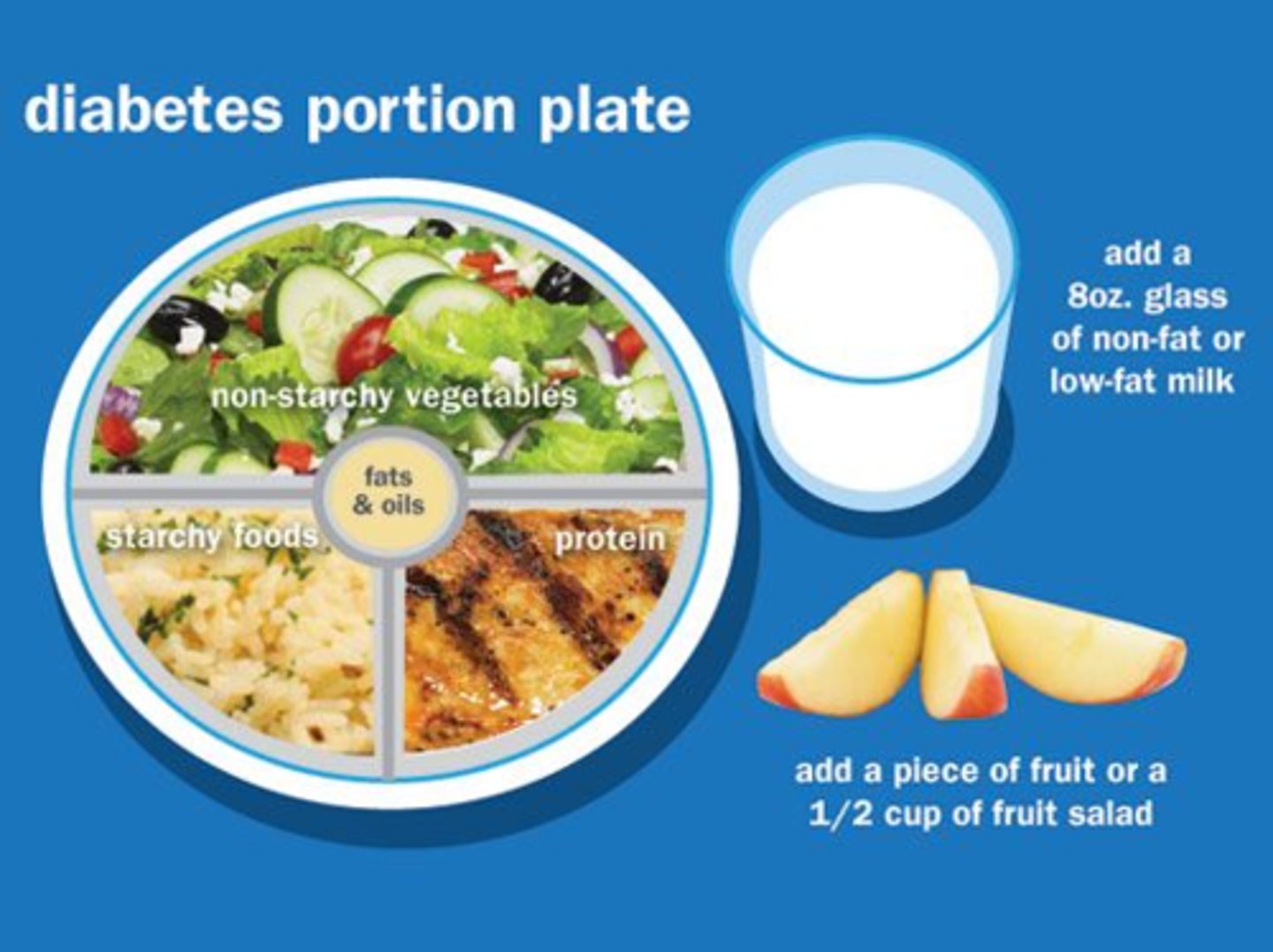
What else do we need to measure other than carb intake for bolus dosing?
- regular meals & snacks (eg. Bedtime snack!!!)
- low glycemic index foods (release glucose more slowly) & protein intake
- regular activity/exercise (usually lowers BG)

What is a BOLUS of insulin?
- Rapid or Short acting Insulin pre-meals (3x per day)
- Approximate estimate from original calculation
- Pre-meal BG check
- Estimated meal-carbohydrate content !!!
- Give BOLUS pre-starting the meal
- CHECK BLOOD GLUCOSE post-meal (eg at peak action) => Goal: 4-8 mmol/L = normal
* Note: newly diagnosed patients will do 8 blood glucose checks per day

How to watch for Insulin correction & titration
- Too high (BG >10) = require more insulin for that
- Too low (BG <4) = too much insulin

What causes high ketones to be present?
- present due to insufficient insulin-glucose transport => causing fatty acid breakdown into ketones for energy
How to measure urine ketones
- with a urine ketone strip
- monitor levels (range of negative => trace => large)
- if rising ketones = Intervene!
- note* high ketones seen in ketoacidosis (diabetic ketoacidosis = DKA)
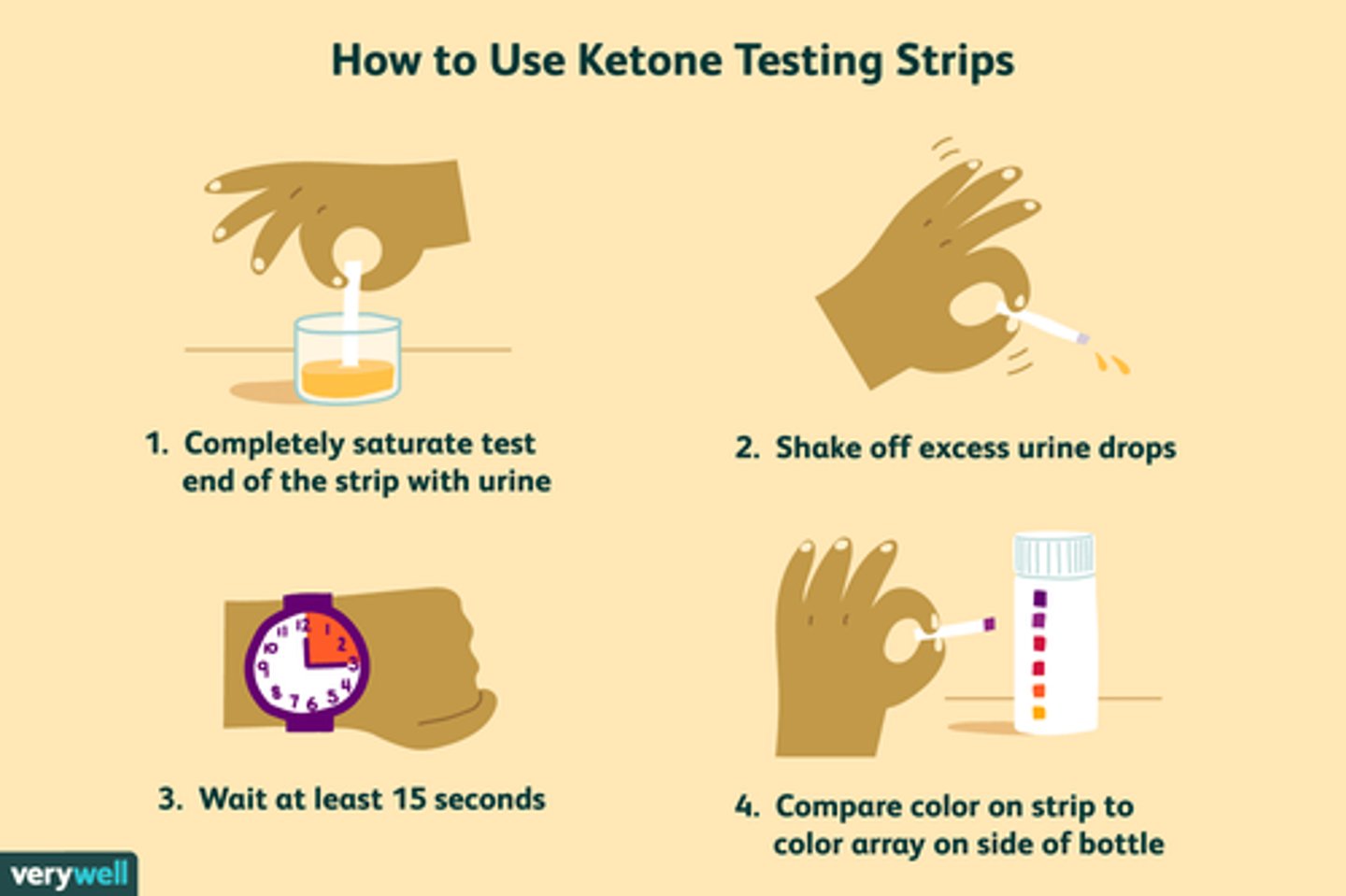
What is the sliding scale?
- BG monitoring, use of glucometer
- regimen (Rx) for rapid-acting or short-acting insulin
- rx in pts chart
- schedule: TID-QID
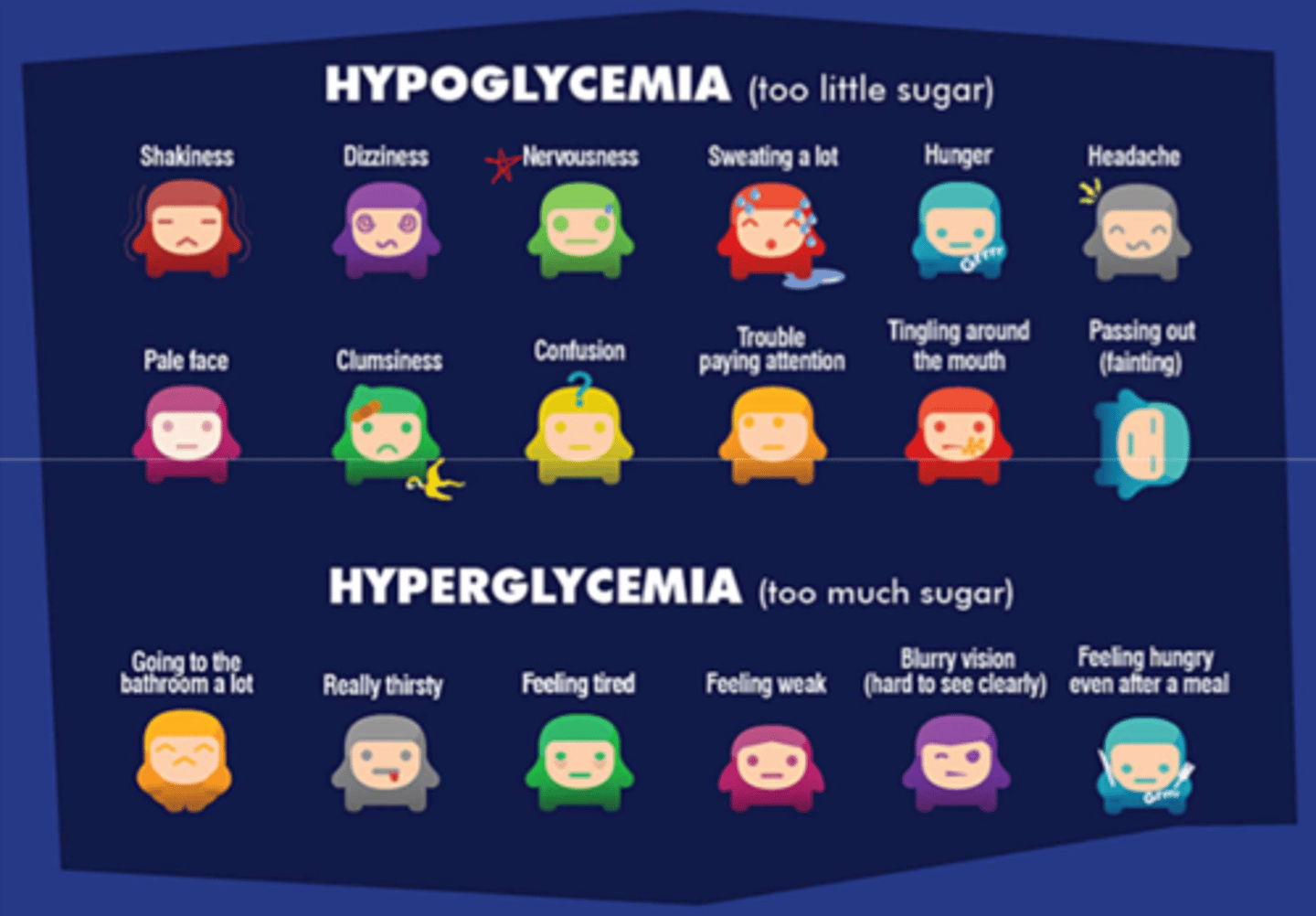
What are the most telling signs of hypo vs hyperglycemia
- hyperglycemia: fatigue, thirst (takes a while to build up! dangerous long term but not fast onset!)
- hypoglycemia: loss of focus, nervousness, shakiness (rapid onset, much faster than hyperglycemia!!)
**if your patient seems 'off' = check blood glucose level
What can cause sugar imbalances
- illness => increases sugar levels due to cortisol release, SNS stimulation
- high stress releases catecholamines & cortisol, causing increase in lysis of stores
- exercise => decreases sugar levels

What is hyperglycemia?
- high plasma glucose (high osmolality)
- shift of potassium out of cells => ECF => excreted (results in VERY low K+)
- low cellular function, ketone accumulation => DKA ! (renal failure may present as an outcome of this!)
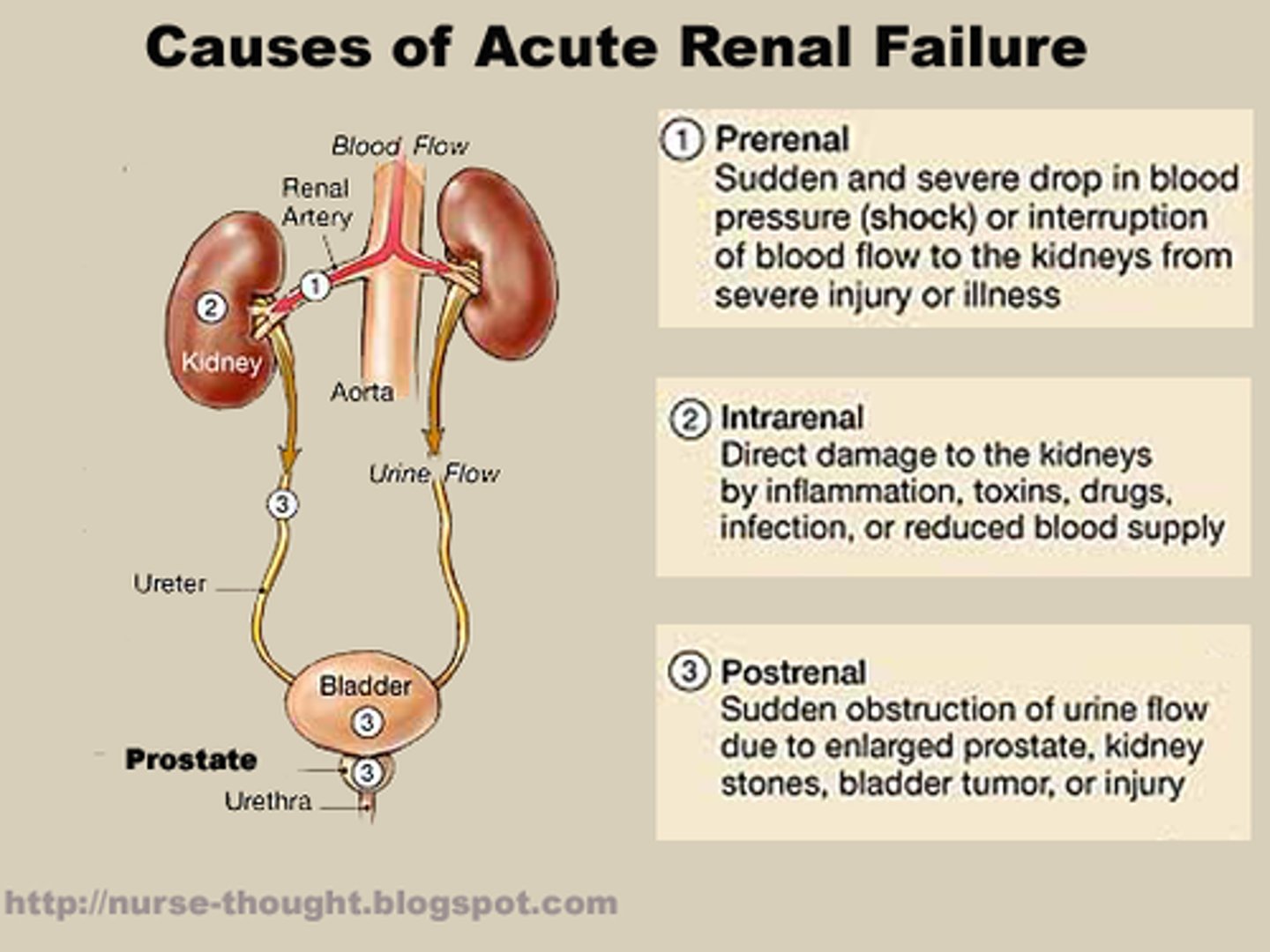
what is a sign in DKA that causes HIGH K+?
- acute renal failure!!
- patient will present with high K+, even though hyperglycemic pts usually present with low K+)
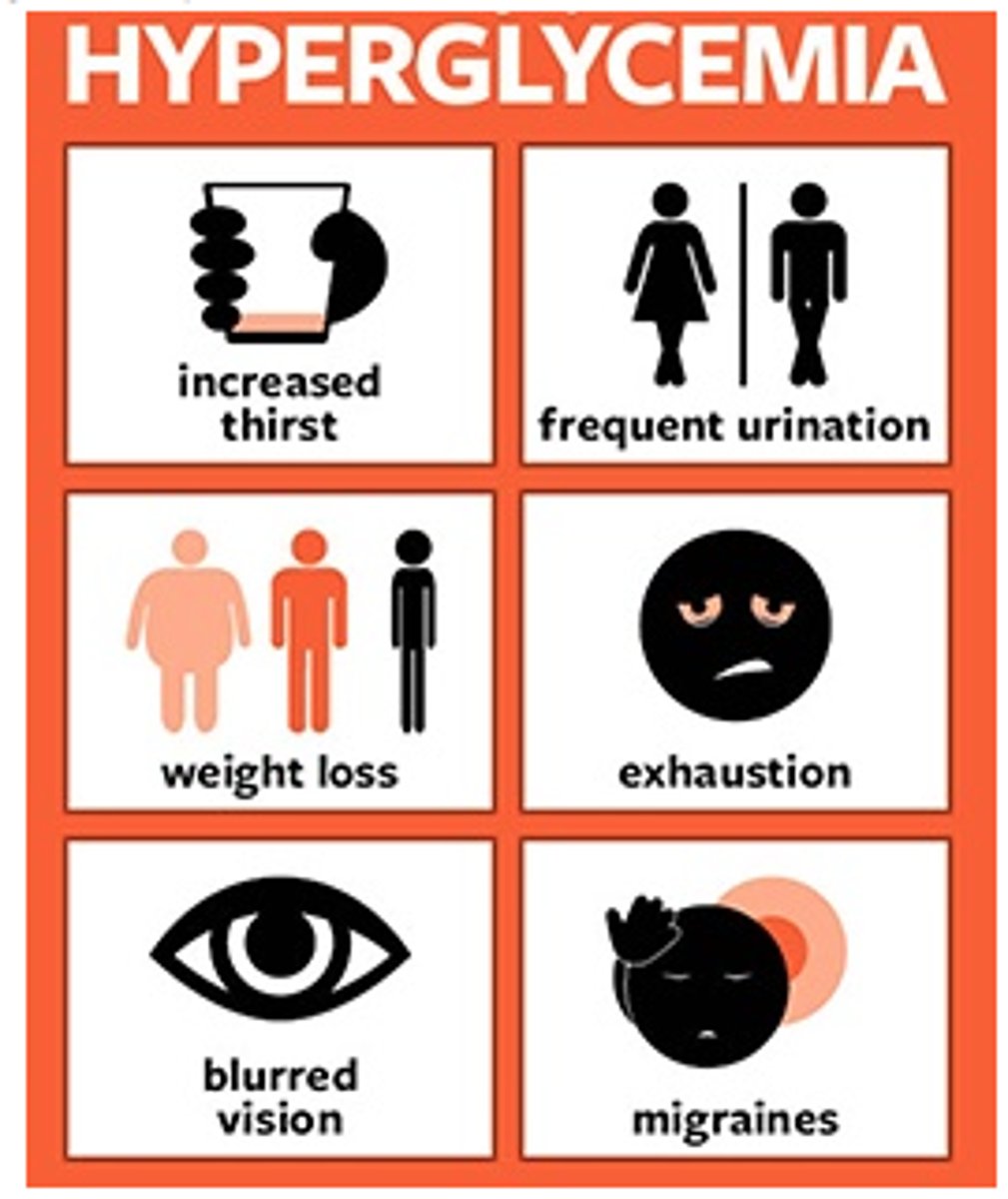
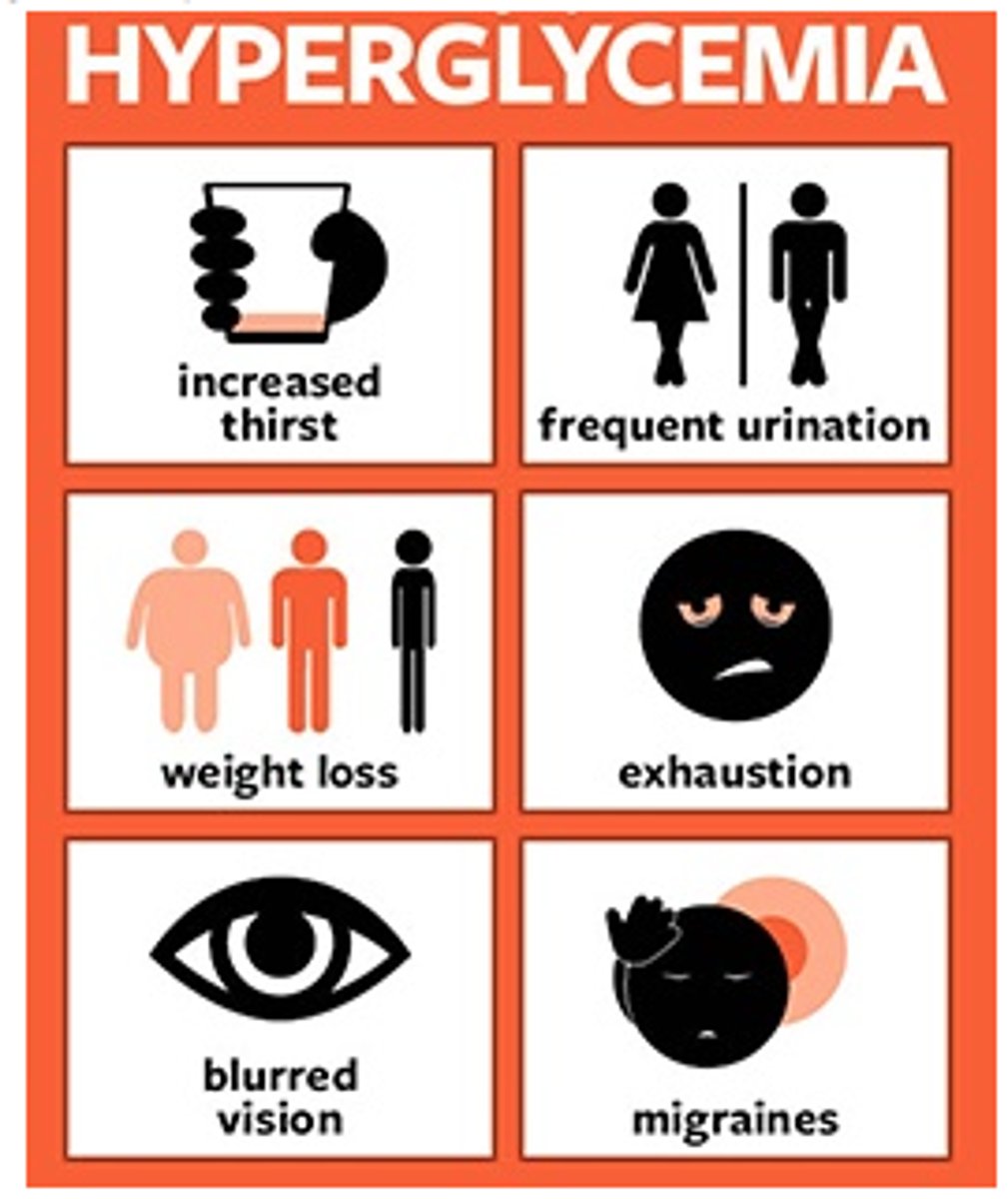
visible s&s of hyperglycemia
- diuresis, excessive thirst (to try and dilute the sugar)
- fatigue, weakness
- blurry vision
- hunger even after a meal
visceral s&s of hyperglycemia
- high BG, high urine ketones + glucose in urine
- acetone (fruity) breath
- high lactate (metabolic acidosis)
- changes in LOC, N&V
- Kussmaul resps (irregular w/ long expiration to try and blow off excess byproducts)
How to tx hyperglycemia?
- regular insulin IV, IV fluids, KCl, close monitoring (Gl q1h)
- additional: neutralize metabolic acidosis (Sodium Bicarbonate)
What is hypoglycemia?
- low BG, most frequent complication in community settings
- danger of night hypoglycemia
- causes: diet change (low carbs), activity (higher than anticipated), insulin (too much)
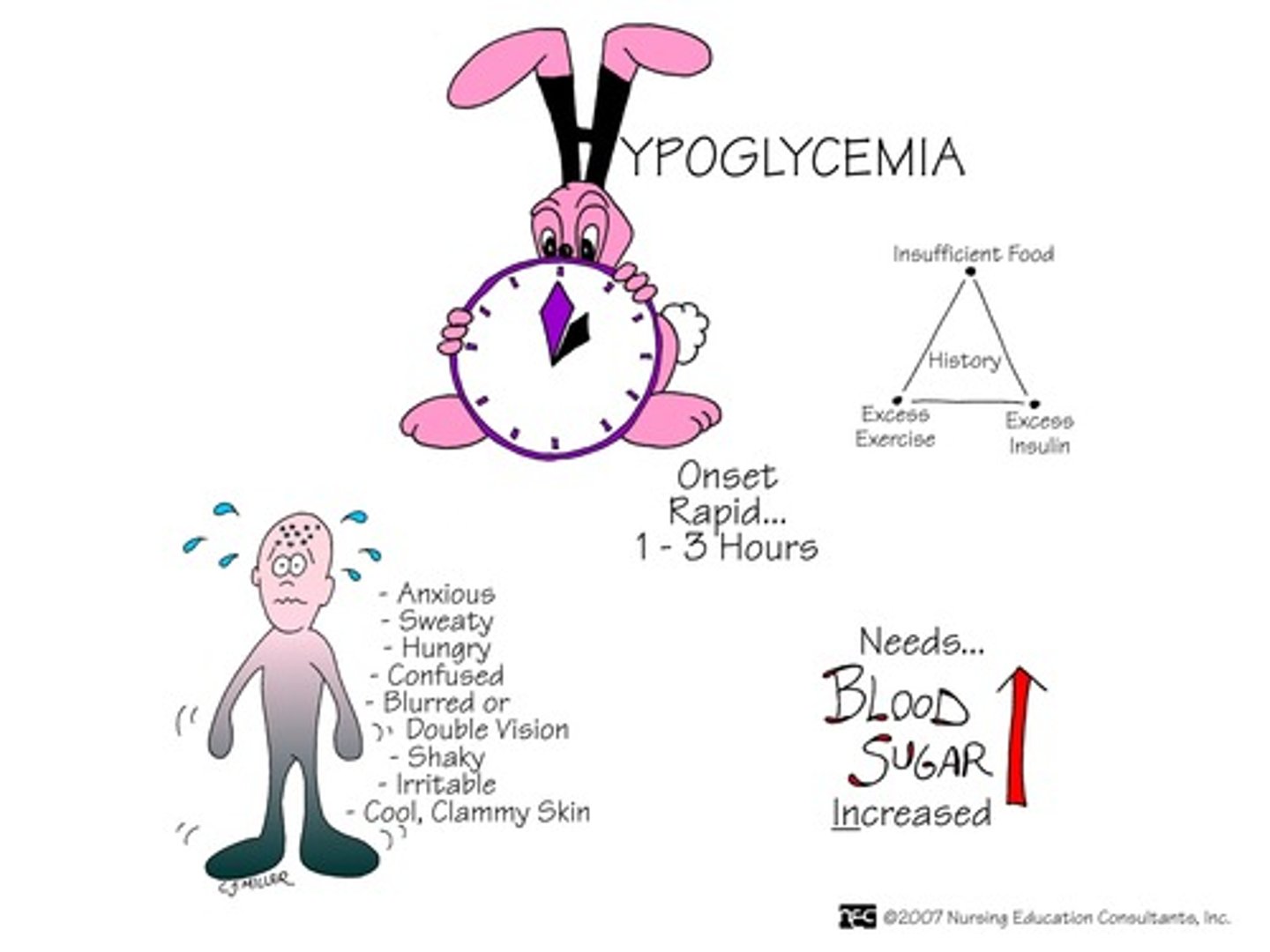
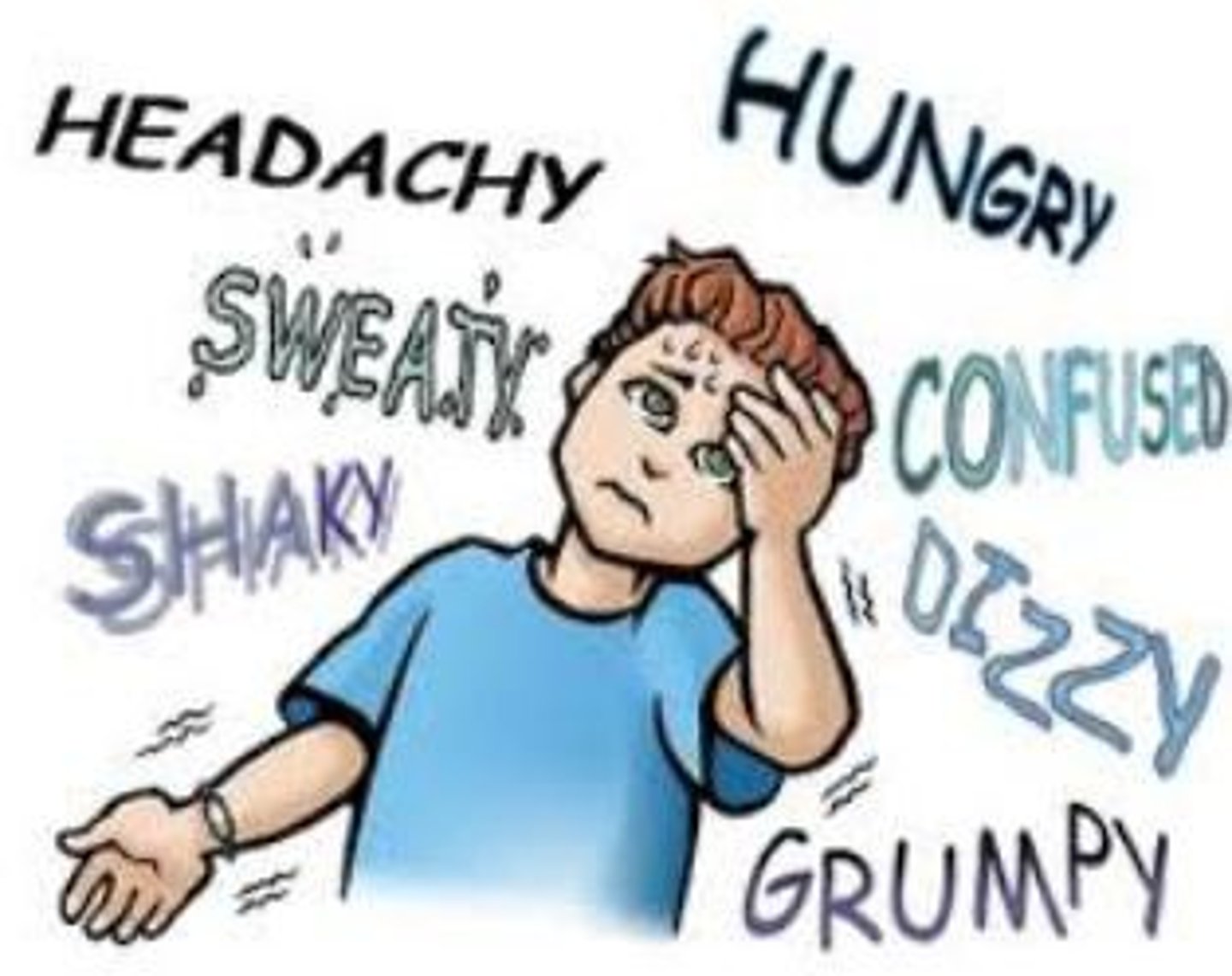
s&s of hypoglycemia
- weakness, tachycardia, tremors, nausea, thirst/hunger, diaphoresis, headache

How to tx hypoglycemia?
- tx: glucose! => tx form depends on LOC
- Conscious: glucose gel/tablet 15g x4; apple juice
- Unconscious: 50% dextrose IV; Glucagon IM

Your diabetic patient seems a bit 'off.' They are experiencing thirst and fatigue. What is this a sign of?
hyperglycemia, which is dangerous long term but not fast onset and takes a while to build up
Your diabetic patient seems 'off.' They suddenly begin experiencing loss of focus, nervousness, and shakiness. What is this a sign of?
hypoglycemia, which is rapid onset
What is the fx of glucagon?
Glucagon stimulates release of glucose from stores
How to keep glucose levels even in daily life?
- blood glucose monitoring
- food intake monitoring (low glycemic index, controlled carb intake (increase fibre), hydration)
- exercise
- control stress levels

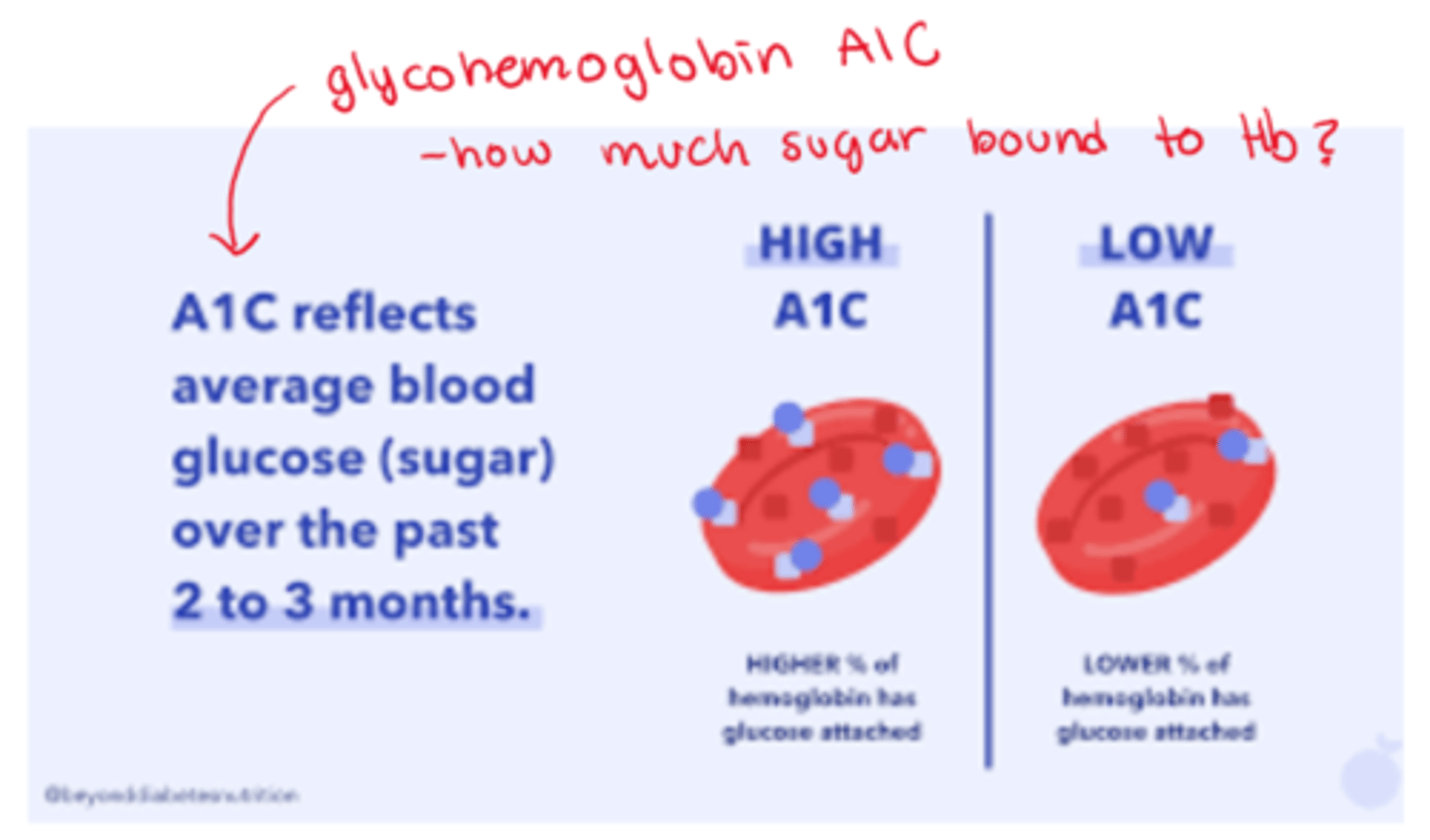
How are glycohemoglobin tests effective?
- glycohemoglobin (A1c hemoglobin) => serum test of glucose bound Hb
- assessment of long term glucose control (eg. over 3 months)
- a tool to monitor DM patients & dx patients at risk for DM
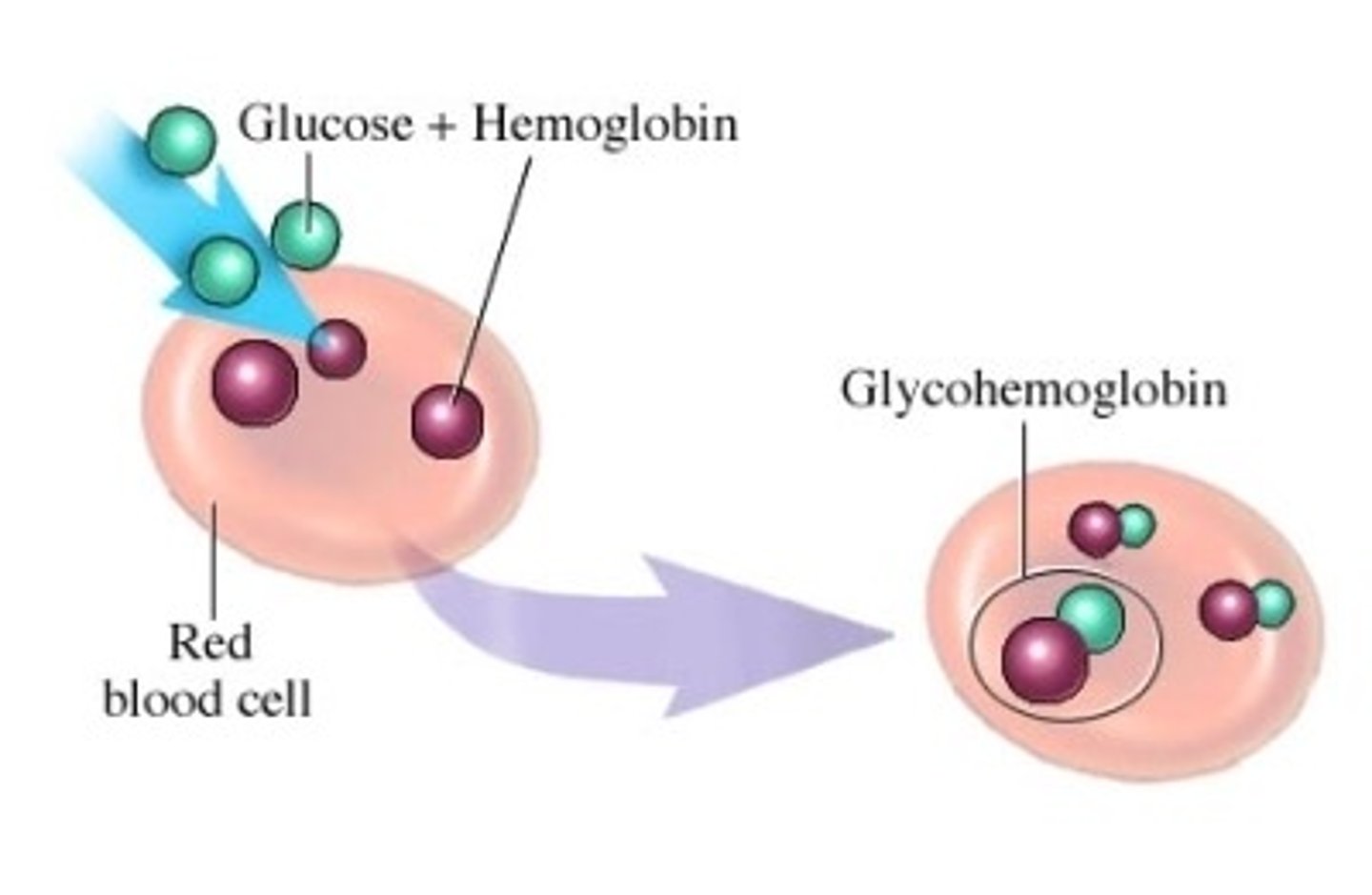
What is the difference between high A1c and low A1c
- high A1c: higher % of Hb has glucose attached
- low A1c: lower % of Hb has glucose attached