MCAT Organic Chemistry Review
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
How do we name alcohols when they are the highest priority group? What if they aren’t?
If the alcohol is the highest priority group, the molecule drops the -e and gets an -ol suffix.
If it is not the highest priority group, then it is named as a substituent using the prefix hydroxy-.
What do ortho, para, and meta mean?
Ortho means the functional groups are on adjacent carbons of a benzene ring. If the groups are separated by a single carbon, then they are meta. If they are on opposite sides of the benzene ring, they are para.
How to turn a primary alcohol into an aldehyde?
Add pyridinium chlorochromate (PCC)
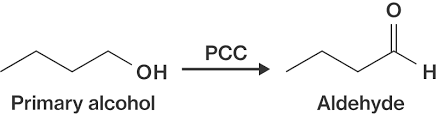
How to to turn a secondary alcohol into a ketone?
Add PCC or a strong oxidizing agent like chromium VI, or sodium or potassium dichromate salts (Na2Cr2O7, K2Cr2O7).
How to turn a primary alcohol into a carboxylic acid?
Add a strong oxidizing agent like chromium VI, or sodium or potassium dichromate salts (Na2Cr2O7, K2Cr2O7).
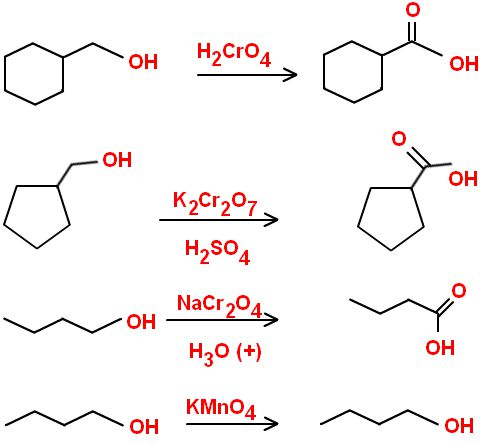
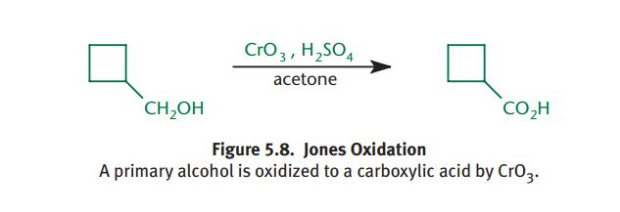
What is the Jones Oxidation?
Chromium trioxide (CrO3) is dissolved with dilute sulfuric acid and acetone. This is a very strong oxidizing agent and it turns primary alcohols into carboxylic acids and secondary alcohols into ketones.
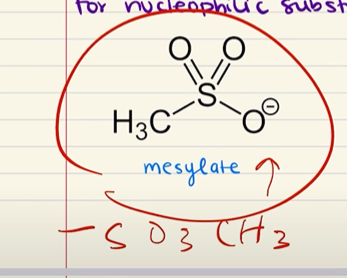
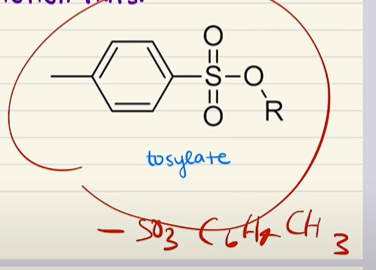
Mesylate
Tosylate
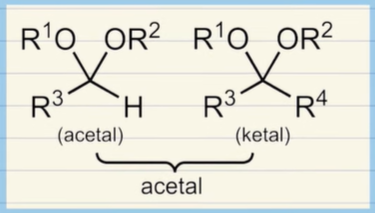
Acetal and Ketal
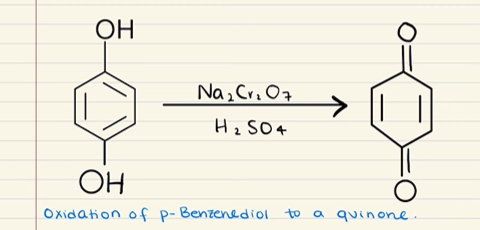
What happens when a phenol is treated with an oxidizing agent?
You make a quinone.
Adding methylsulfonyl chloride or toluene sulfonic acid to an alcohol will…
Create a protecting group (either a mesylate or tosylate) that prevents the alcohol from reacting. This can be removed with a strong acid.