MCAT Chemistry: Atomic Structure and Periodic Trends
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
atomic number
the number of protons in the nucleus of an element determines the element as it does not change.
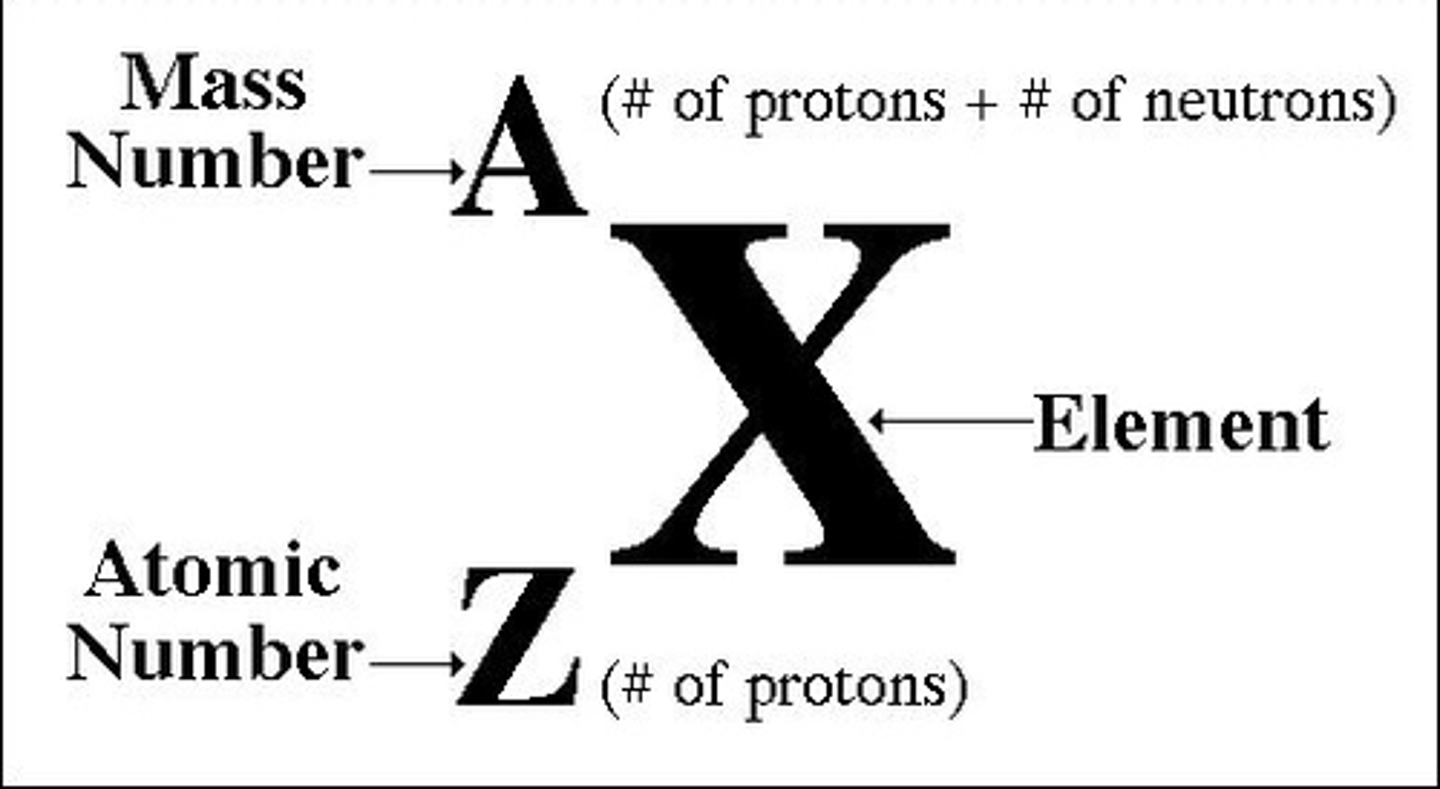
mass number
the number of protons plus neutrons, determines the isotope, and mass of the element
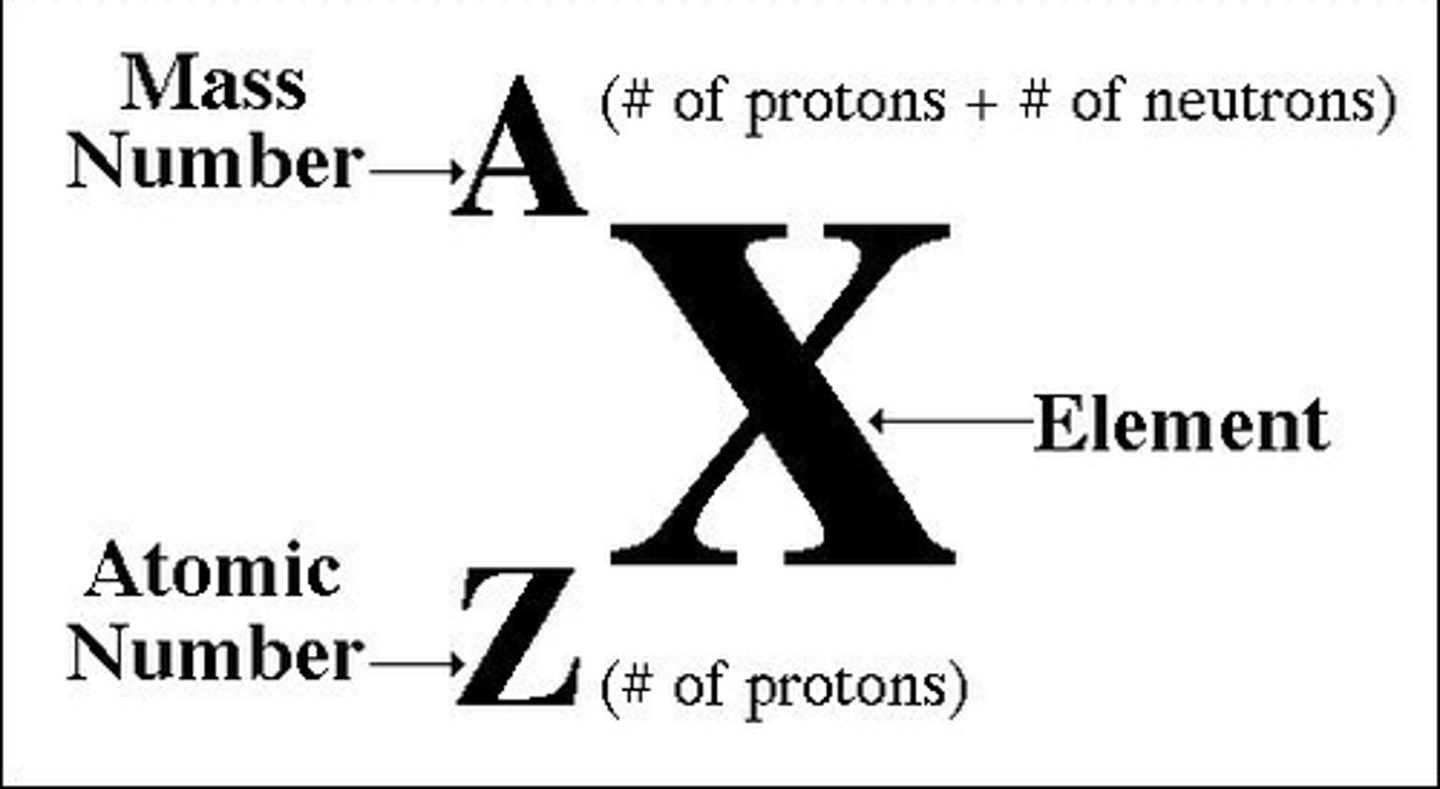
isotope
Atoms of the same element that have different numbers of neutrons
charge of an element
the number of protons minus the number of electrons
distances between orbits from the nucleus
In the Bohr model, the distance decreases with the distance from the nucleus; the energy increases with the distance
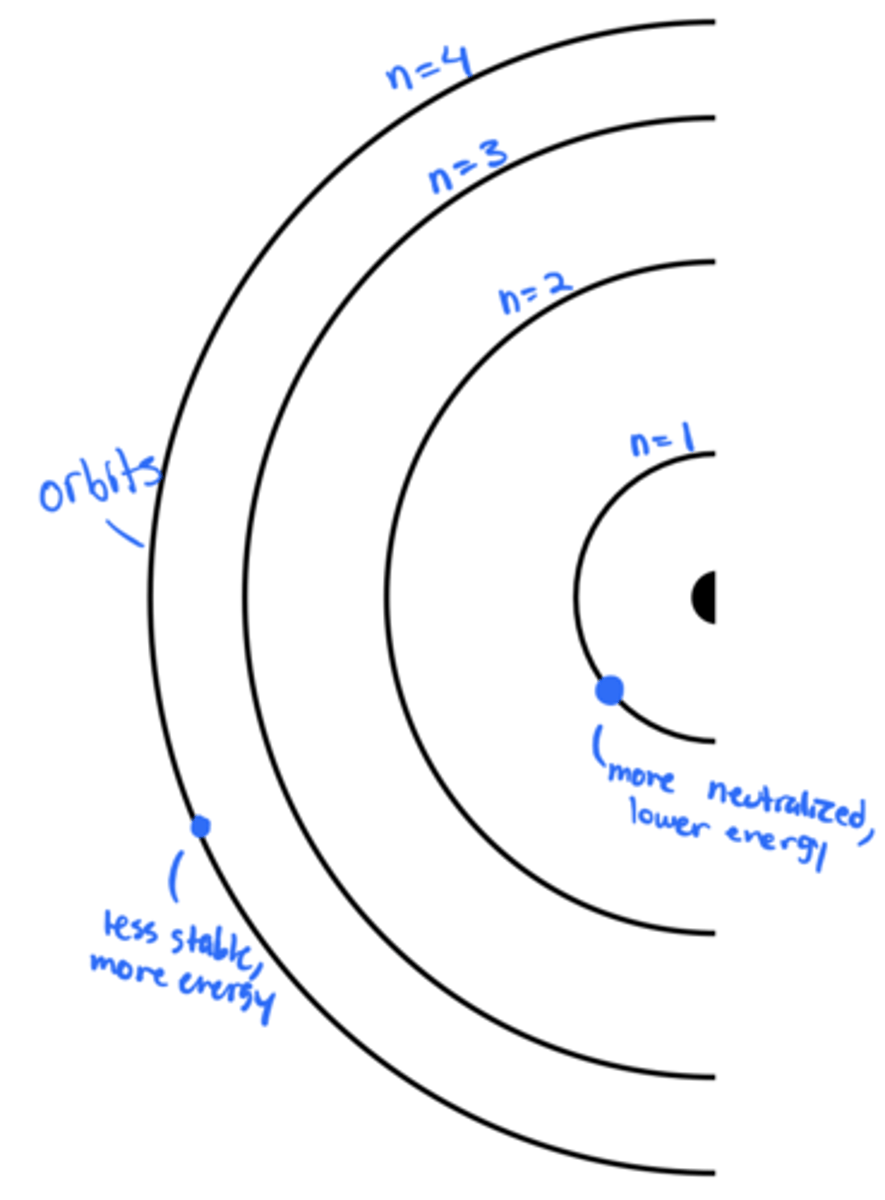
principal quantum number
symbolized by n, indicates the main energy level occupied by the electron
electrons absorb specific quantities of energy that
match the energy difference between an electron's ground and excited states. Electrons are only able to jump up ONE energy level
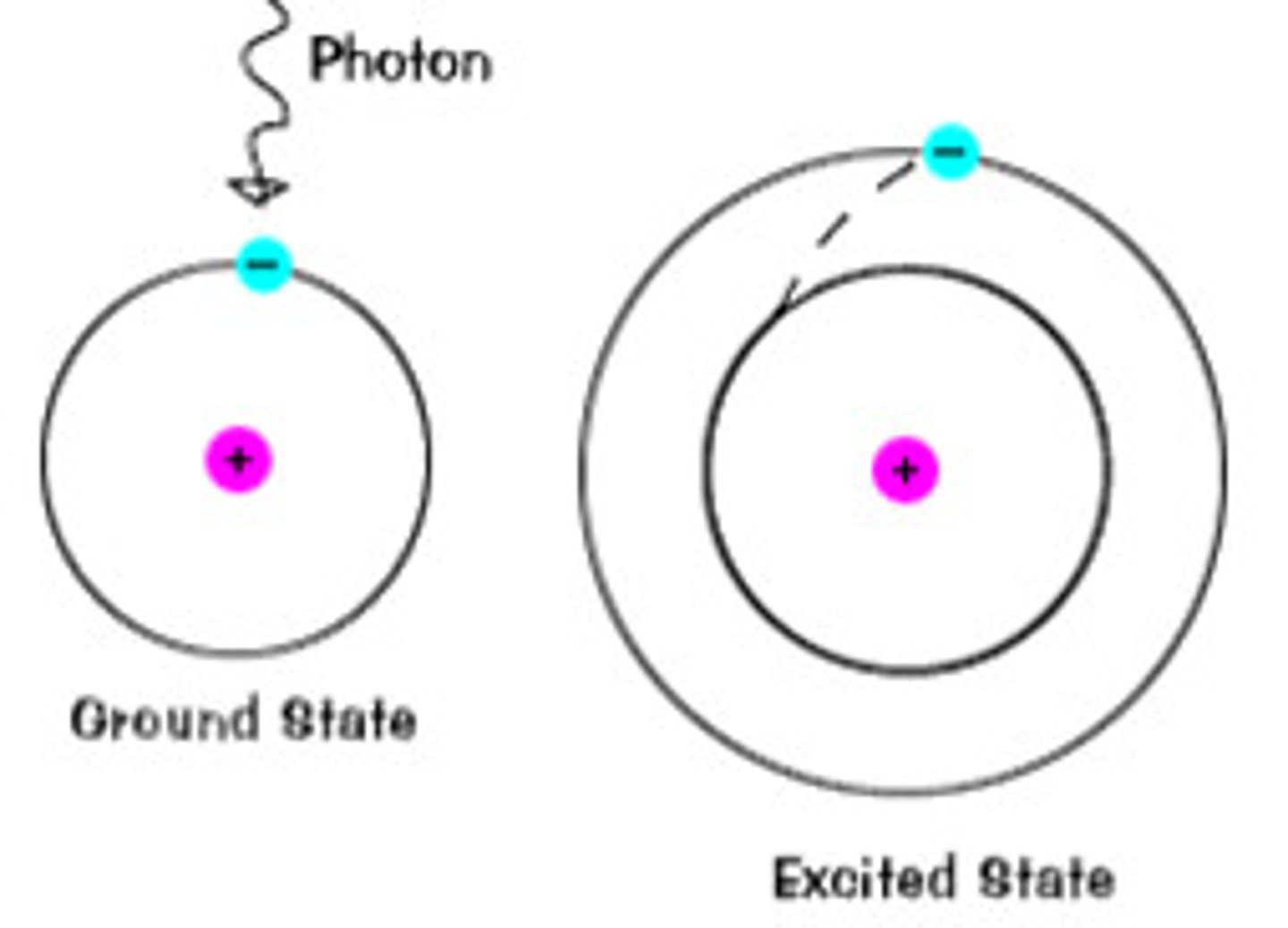
electrons emit a photon of equal energy when
returning to a lower energy state. can occur in one step or multiple. the bigger the energy difference between energy levels, the higher energy the photon emits
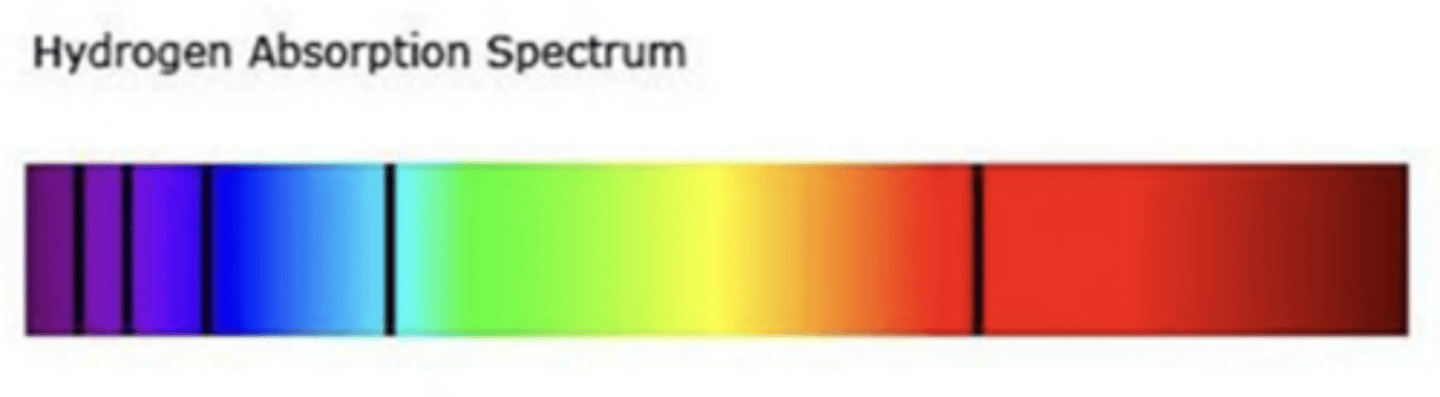
hydrogen absorption spectrum
Black lines indicate the wavelengths of light that are absorbed
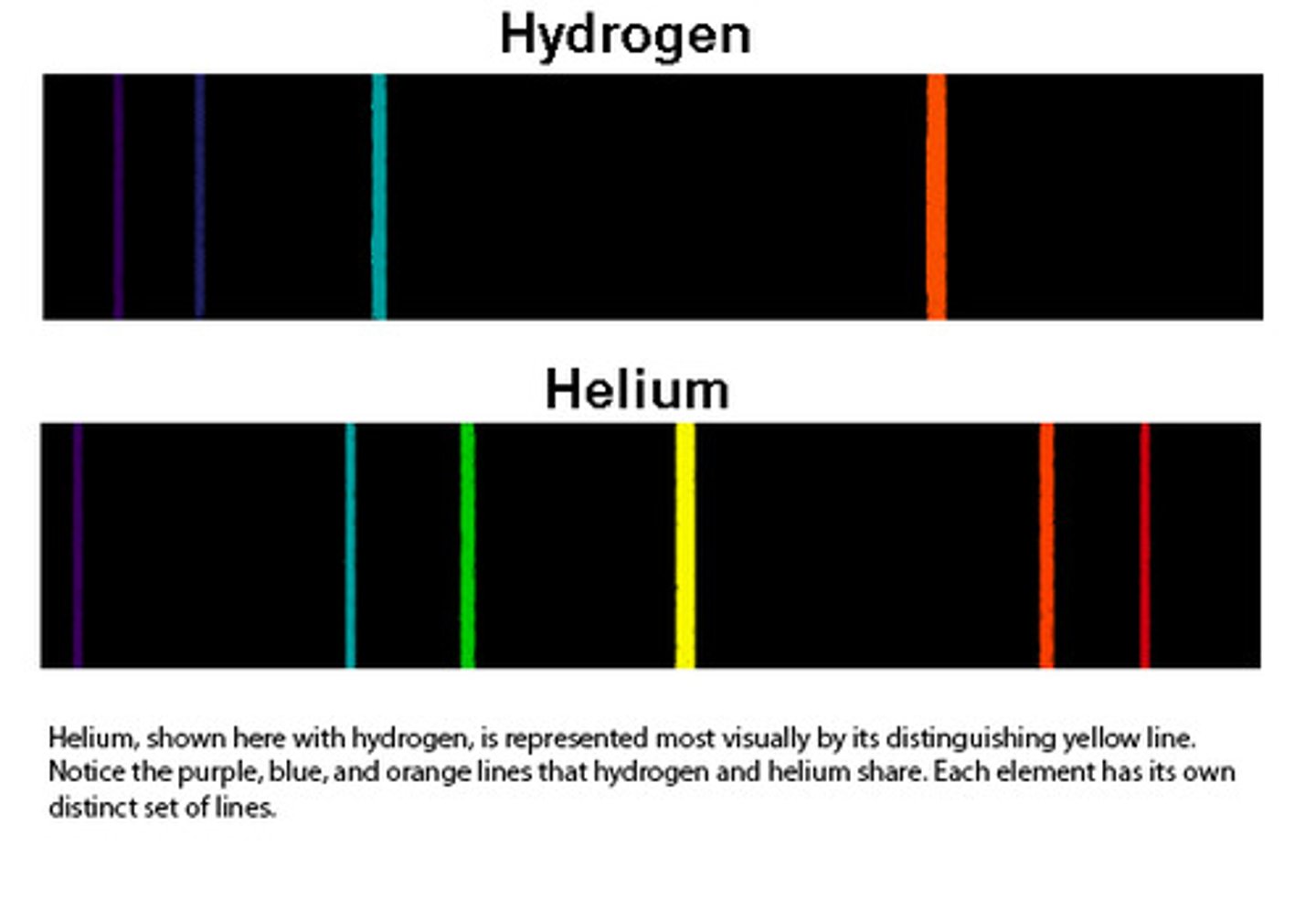
Hydrogen Emission spectrum
Color lines indicate the wavelengths of light that are released
energy of a photon
E = hf = hc/λ
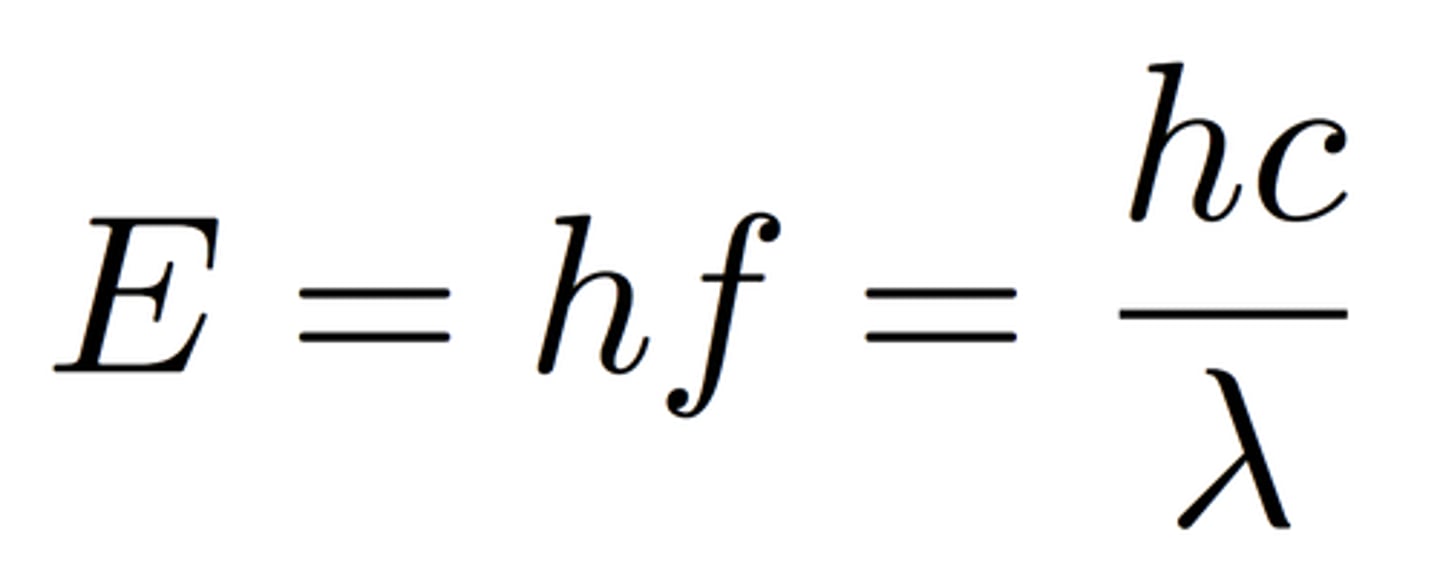
electromagnetic spectrum
shown in decreasing energy, decreasing frequency, and increasing wavelength
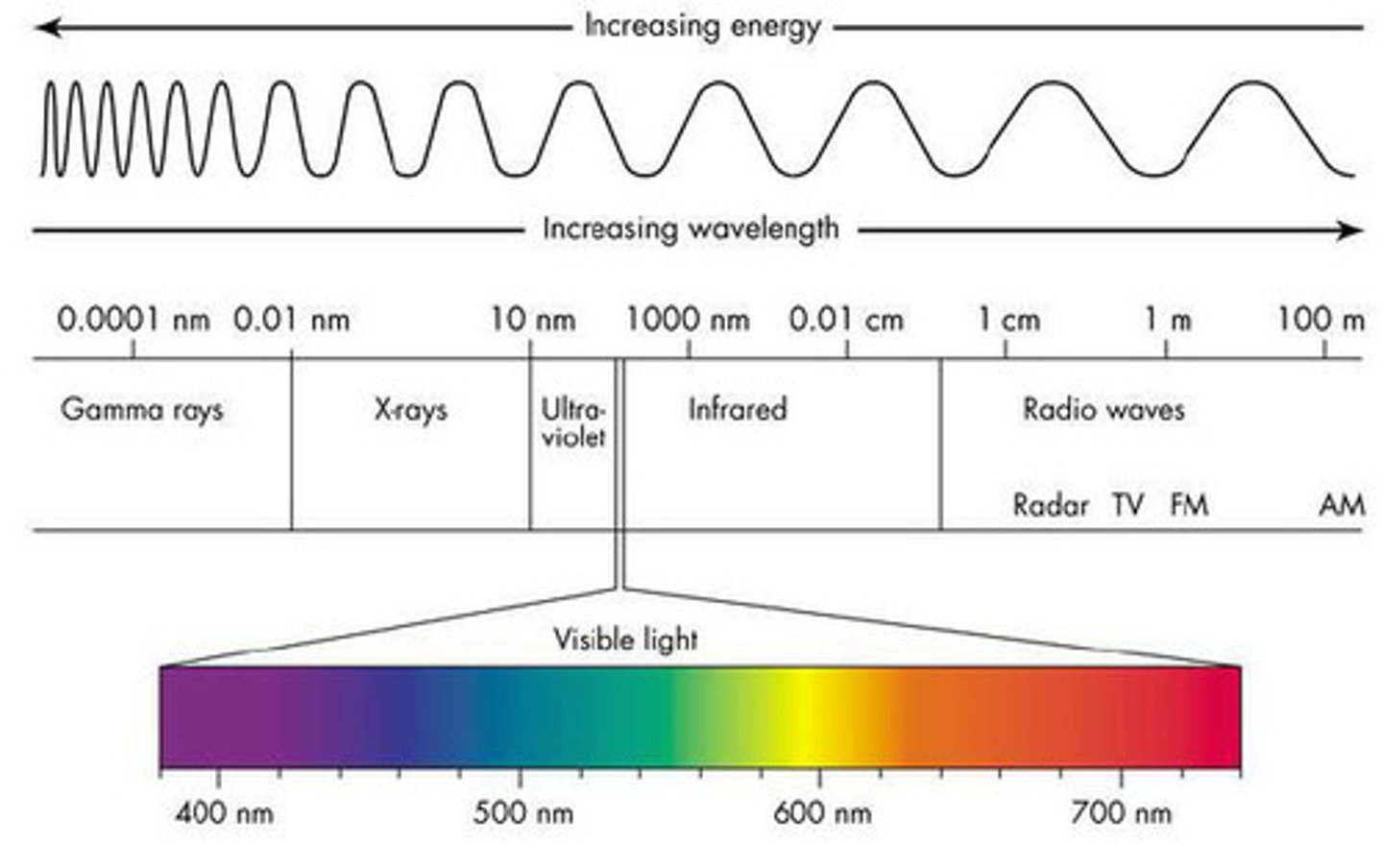
electron energies are quantized
energy increases with distance from the nucleus and with the complexity of the orbital shape
energy shell
group of electron orbitals that share the same energy level; each period is higher than the last
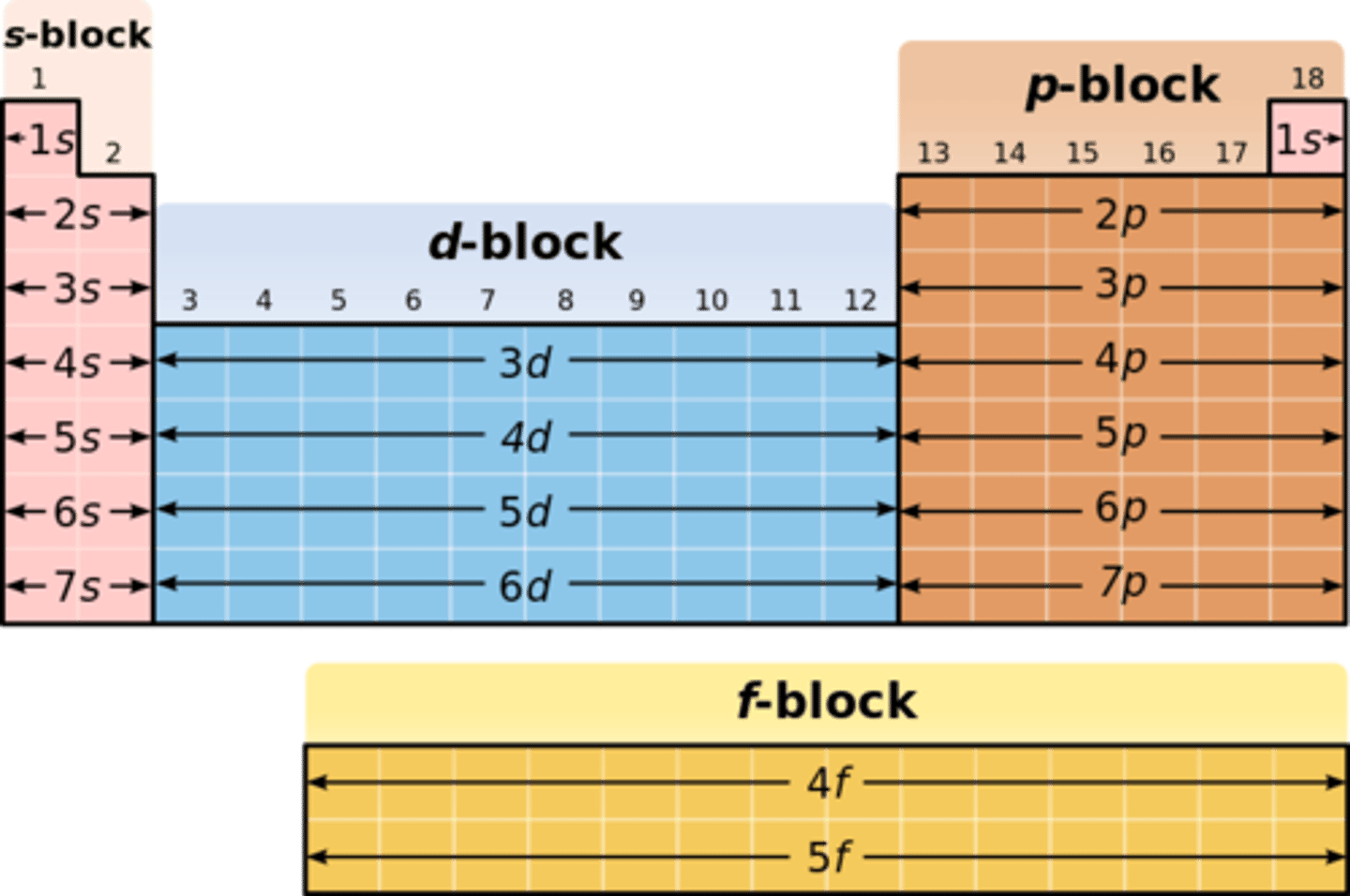
energy subshell
"energy sublevels"; the set of orbitals in a given shell; the orbitals of this all have identical, or similar, shapes. determined by blocks on the periodic table, each subshell is higher energy than the last.
Pauli principle
atomic orbitals can hold only 2 electrons at most, and they must have opposite spins
Aufbau principle
An electron occupies the lowest-energy orbital that can receive it. electrons are first removed from valence orbitals from highest to lowest energy
exception to Aufbau principle
3d is higher in energy than 4s; 4s electrons are removed BEFORE 3d electrons
Hund's Rule
orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin
paramagnetic
Atom or substance containing unpaired electrons is consequently attracted by a magnet.
diamagnetic
all electrons are paired
ground state electron configuration for ions
write the configuration of the atom, THEN add or subtract electrons
closed shell configuration
an atom having a filled valence shell, stable
Chromium electron configuration
[Ar] 4s1 3d5
Copper electron configuration
[Ar] 4s1 3d10
excited state configuration
must have higher energy than the ground state configuration, must have the correct total number of electrons, must be in any orbital that exists
Alkali metals
Group 1 tends to lose one electron
Alkaline Earth Metals
Group 2 tends to lose two electrons
effective nuclear charge
the positive charge that an electron experiences from the nucleus, equal to the nuclear charge but reduced by any shielding or screening from any intervening electron distribution
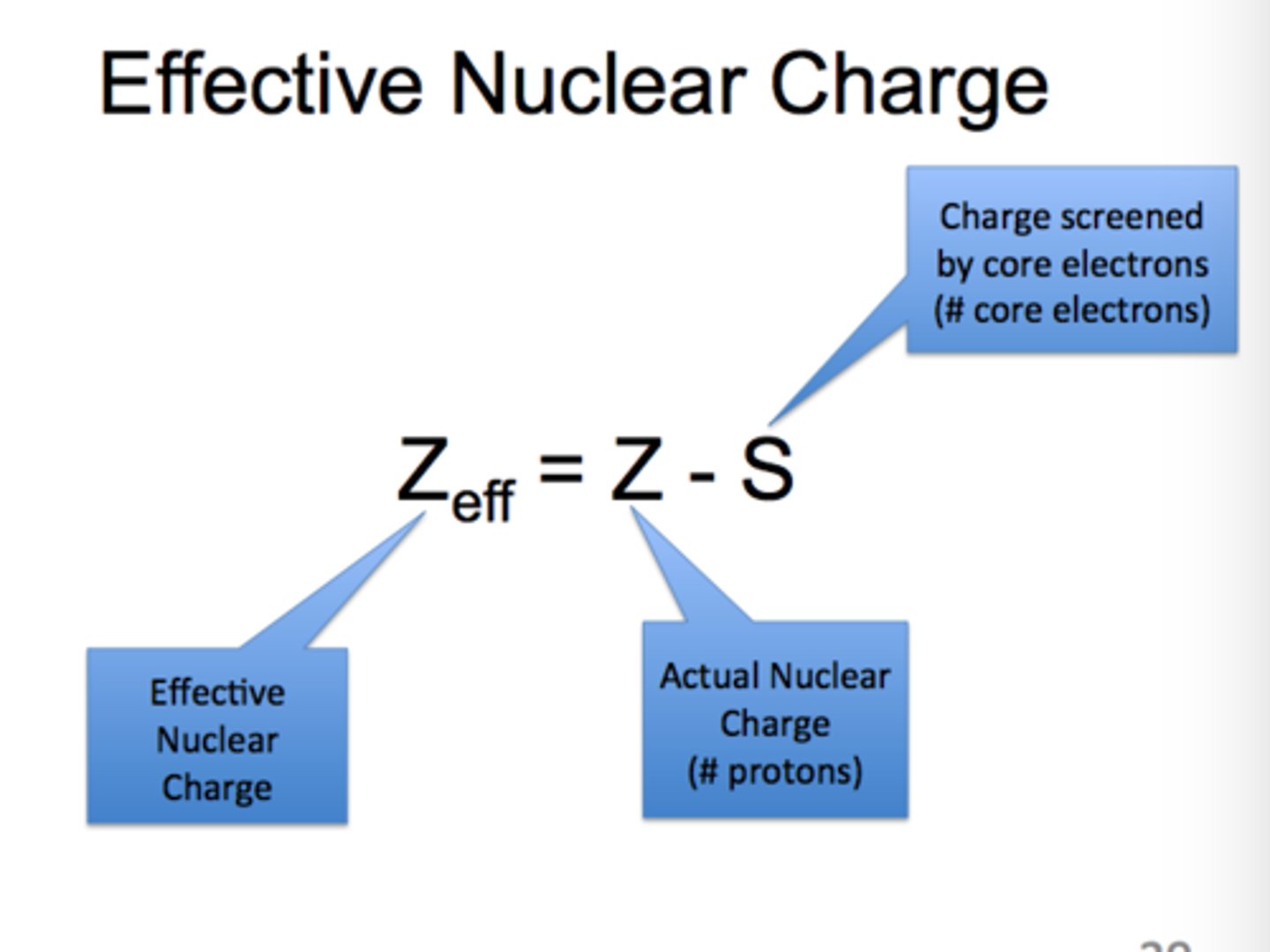
electrostatic force between valence electrons and the nucleus
decreases with increasing shells (down a group), increases with increasing protons (across a period), and decreases with increasing electrons (negative charge)
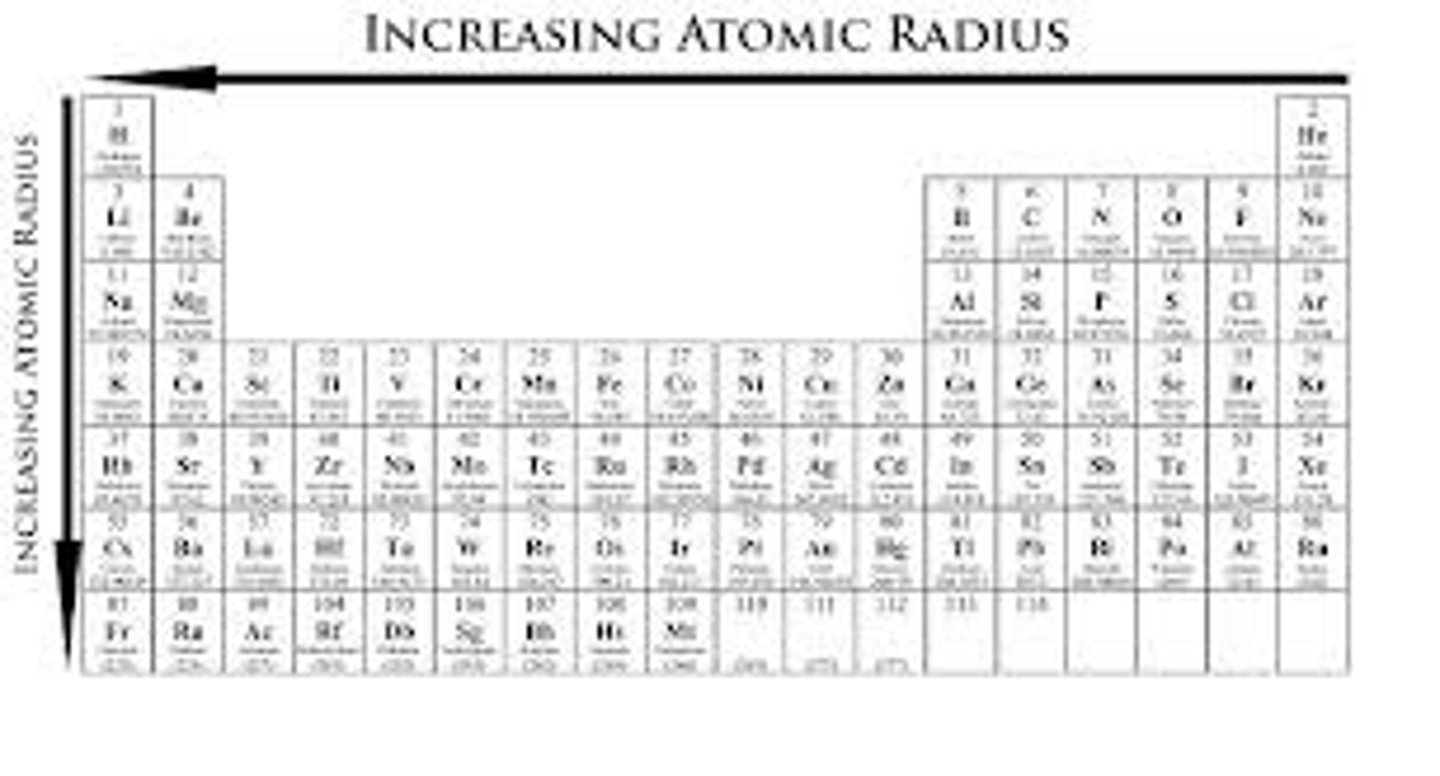
atomic radius
as electrostatic force increases, the atomic radius decreases
ionic radius
the ionic radius increases with increasing negative charge
ionization energy
the energy required to remove a valence electron from an atom in its gaseous state. As electrostatic force increases, ionization energy increases
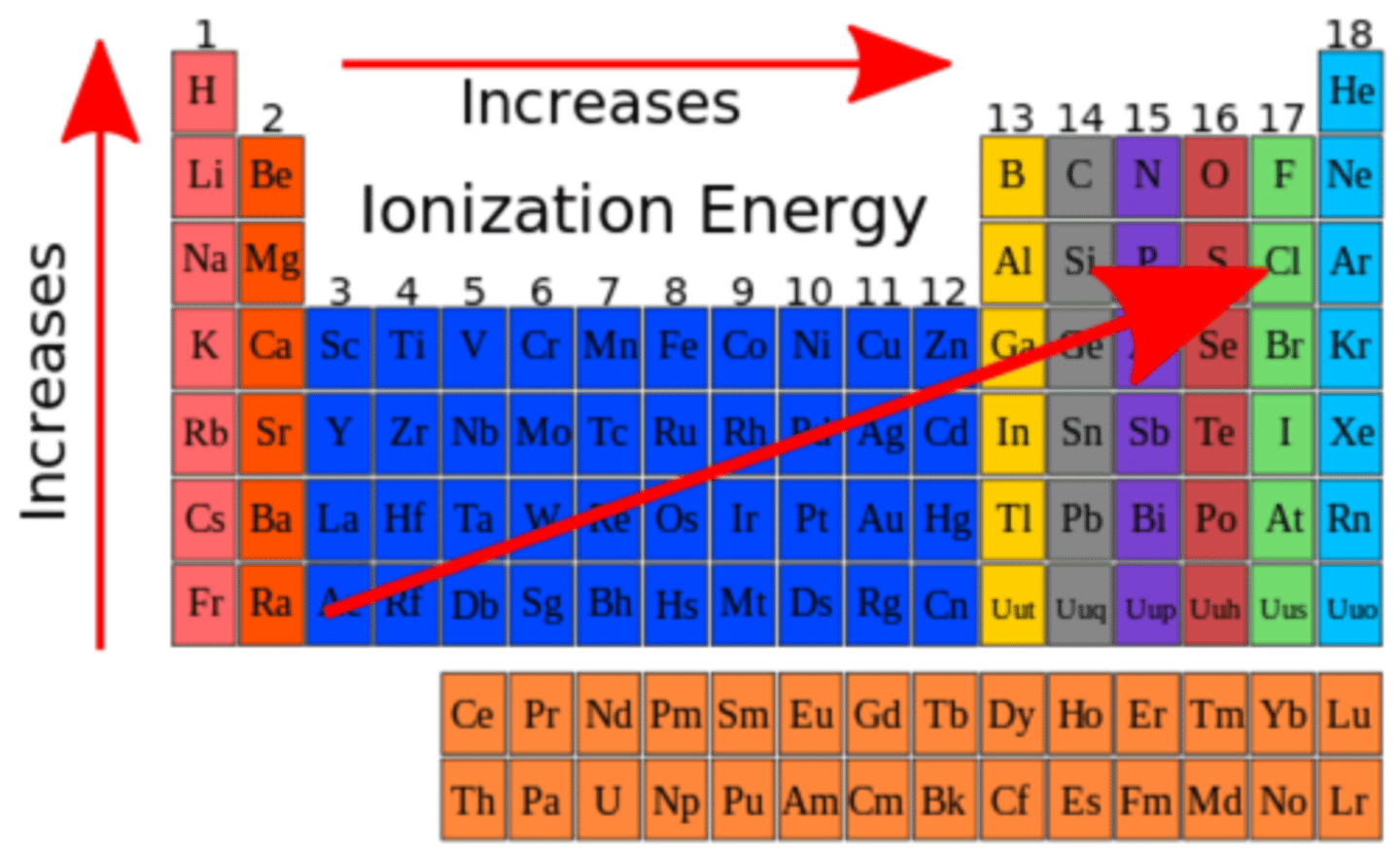
multiple ionization
as the positive charge on an ion increases, so does the ionization energy. it takes the most energy to remove a closed shell/subshell
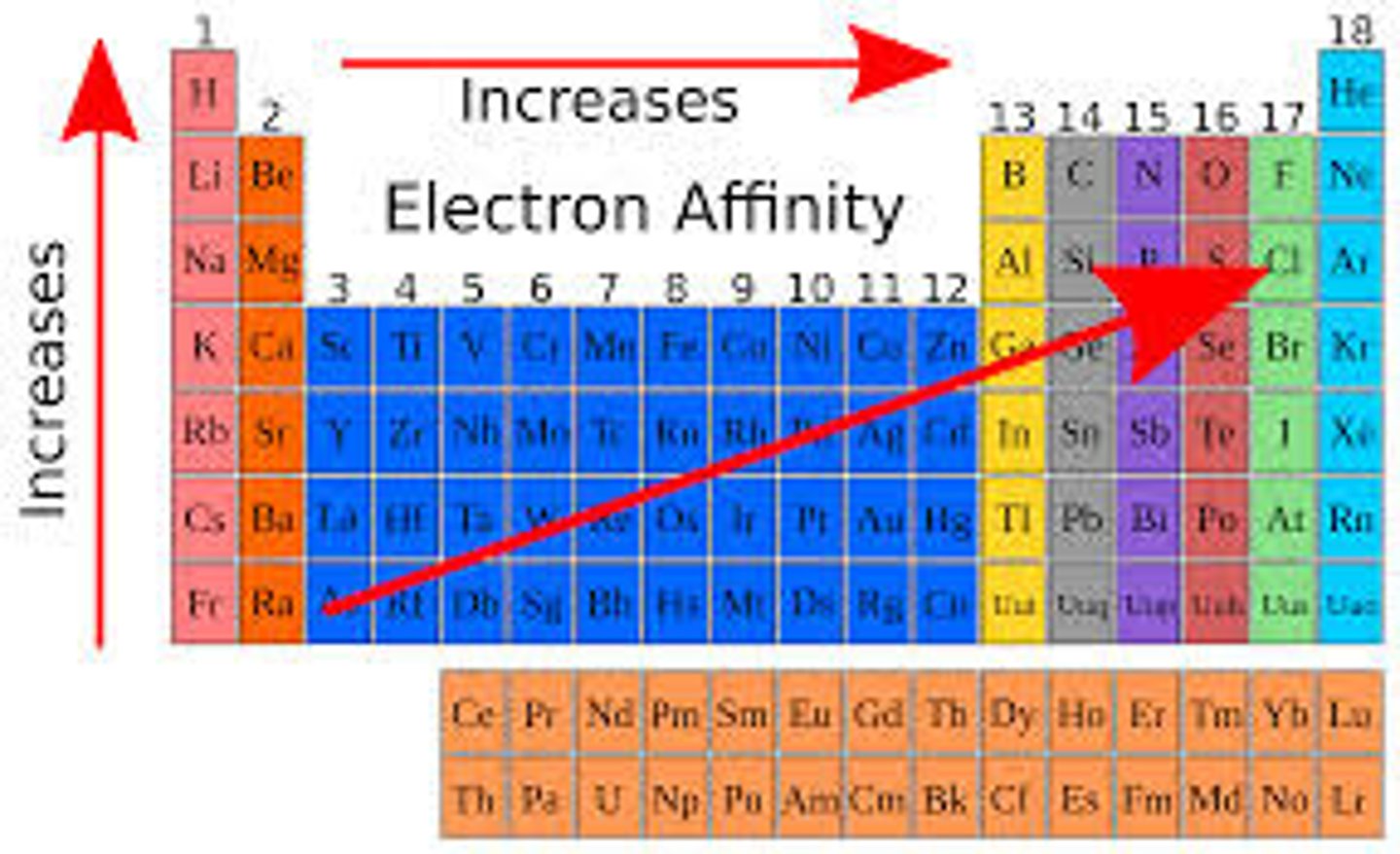
electron affinity
the energy change when adding an electron to the valence shell. As electrostatic force increases, additional electrons release more energy. inert elements require energy to add an electron
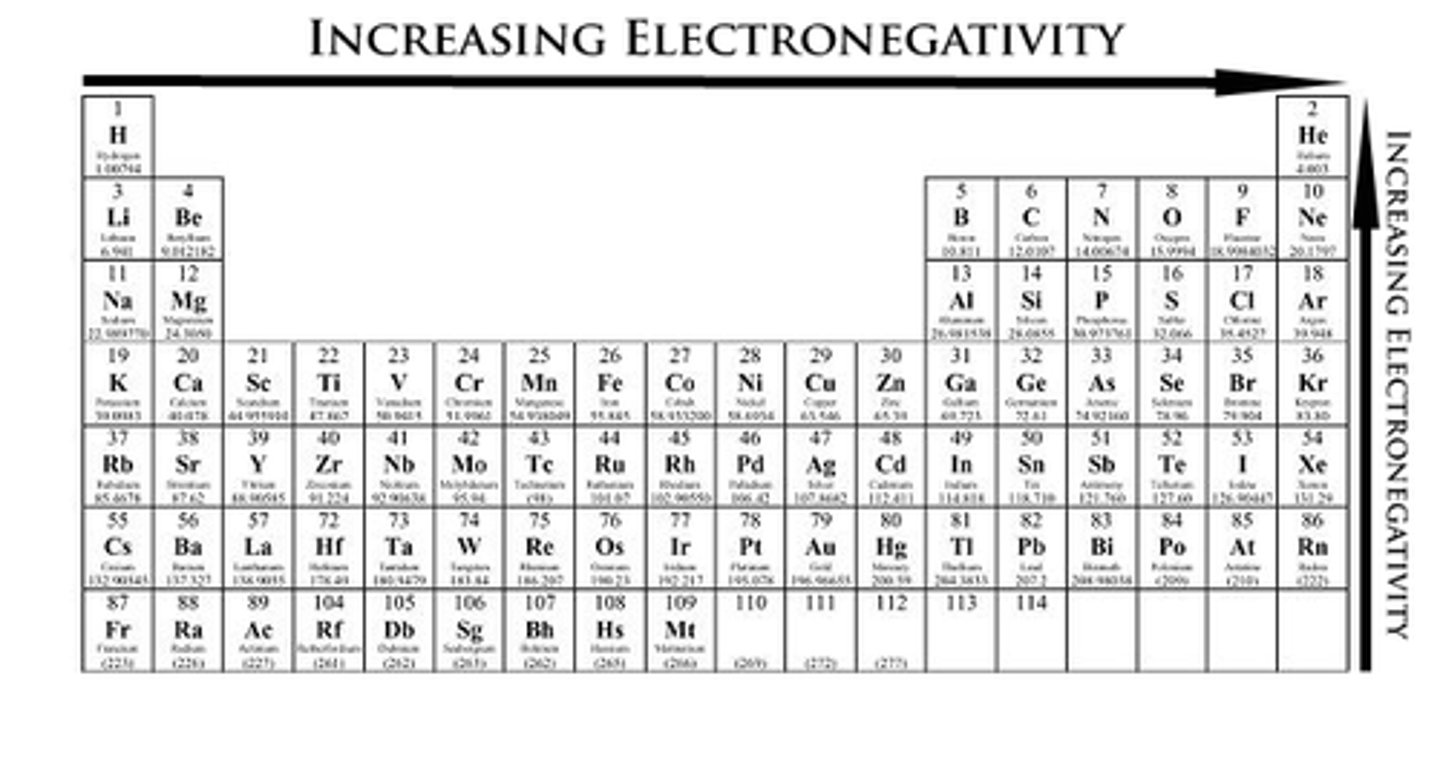
electronegativity
ability of an atom to attract electrons to itself. As electrostatic force increases, the electronegativity increases
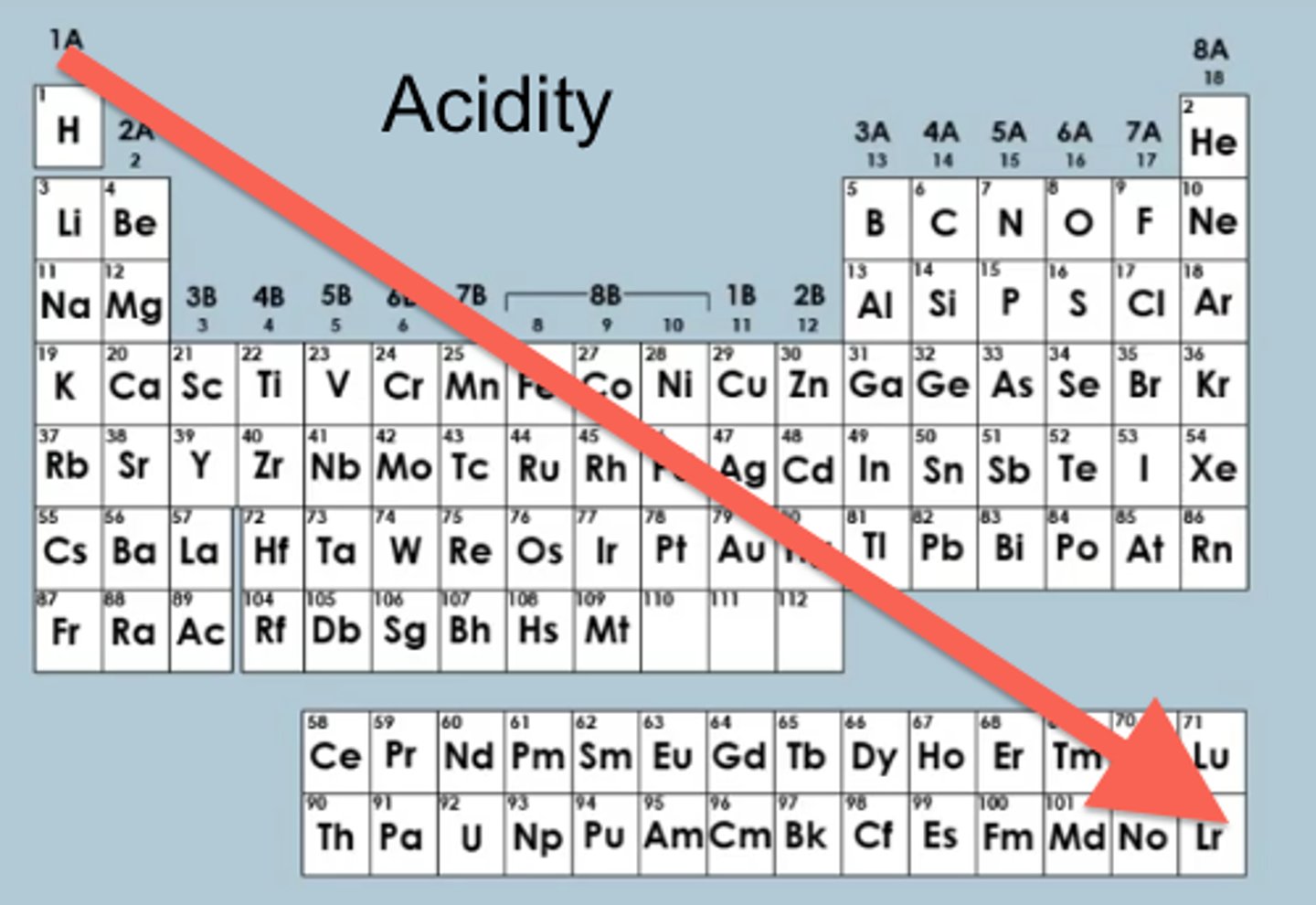
acidity
measure of a compound's ability to donate a proton. As the stability of the conjugate base increases, the acidity increases