Inflammation and Coagulation MDRs, Thrombosis MDR, and Cornell Coagulation Videos
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
110 Terms
Tissue Factor
Constitutively expressed in most cells within the body
Highest concentrations in the brain and endothelial cells
What happens when tissue factor is exposed to the blood stream?
Exposed to FVII which circulates in its active form in the bloodstream
This complex converts FX to FXa which mediates the conversion of FII to FIIa (thrombin)
What stimulates the expression of TF on the surface of endothelial cells and mononuclear cells?
Inflammatory cytokines (particularly IL-6)
Actions of Thrombin in the Inflammation and Coagulation Systems
Facilitates the production of fibrin
Activates FV and FVIII, Protein C, and thrombin activatable fibrinolysis inhibitor (TAFI)
Most potent stimulator of platelets
Can stimulate stimulate leukocytes to produce inflammatory cytokines such as TNF-a, IL-1 and IL-6 through the NF-kB pathway
Can directly stimulate endothelial cells causing them to produce IL-6 and platelet activating factor (stimulates platelets and is a powerful chemoattractant)
Actions of TFPI
Endothelial protein
Main purpose is to decrease coagulation by binding the TF-FVII complex and inactivating it
Inhibits TF and IL-6 to prevent excessive inflammation and coagulation distant from site of injury
Actions of TAFI
Keep fibrin from being broken down (counteracts plasmin)
Inactivates complement 5a which ends up being anti-inflammatory
Action of Protease Activated Receptors (PARs) 1, 3, and 4
Bind thrombin
Action of Protease Activated Receptor (PAR) 2
Can be activated by the TF-VII complex and Fx
What happens once a substrate binds to a protease activated receptor?
The PAR cleaves part of the receptor exposing a neoamino terminus which allows the receptor to become its own ligand
Changes that Occur Once the Platelet is Activated that Propagate Inflammation and Coagulation
Upon activation, P-selectin is exposed on the platelet membrane (never expressed on a non-activated platelet)
Allows the platelet to bind to endothelial cells and upon binding cause the endothelial cell surface to interact with leukocytes
P-selectin can also bind to P-selectin glycoprotein-1 (PSGL-1) on the surface of leukocytes which once bound, causes activation of the target leukocyte, promoting TF expression from the leukocyte as well as reactive oxygen species (ROS) and cytokine release (IL-1, CD 40 ligand)
TLR-4 Receptor on Platelets
Platelets have a TLR-4 receptor which can be activated by LPS
LPS activated platelets tend to migrate to the point of infection and will engage with leukocytes to form neutrophil extracellular traps
LPS activated platelets degranulate and express P-selectin on its cell surface
When this platelet binds to the P-selectin glycoligand (PSGL) on a circulating neutrophil it induces NETosis
Activated platelets can also secrete a damage associated molecular protein - high mobility group box-1 (HMGB-1) which can bind to neutrophil receptors and induce NETosis
NETosis
Leukocytes release neutrophil extracellular traps (NETs) composed of cell free DNA, histones, and antimicrobial proteases to form a web like structure capable of sequestering bacteria and destroying them
NETs can also cause damage to endothelium and surrounding cells causing vascular leakage and edema in the affected tissues
Endothelial Cells in Inflammation and Coagulation
In homeostasis, intact endothelial cells are essential to prevent soluble FVIIa in the blood from interacting with the TF underlying the cells
In patients with severe inflammation, various cytokines such as TNF and IL-1 can induce TF expression on endothelial cells
When tissue disruption occurs, endothelial cells are compromised allowing the FVII in the blood to adhere to TF within the cell or expressed ont eh endothelial cell surface after cell damage
Endothelial Protein C Receptor (EPCR)
Constitutively expressed protein on the endothelial cell surface that is a key regulator in balancing inflammation and coagulation
Binding of this receptor to circulating Protein C vastly increases its conversion to activated protein C (APC) and allows it to exert its anticoagulation and anti-inflammatory effects
EPCR can become soluble and when it does so it can complex with neutrophil proteinase-3 and integrins to block adhesios of leukocytes with the endothelial cells
Thrombomodulin
In homeostasis, this protein is constitutively expressed to bind excessive thrombin away from the site of injury
When thrombin is bound to endothelial thrombomodulin not only does it prevent thrombin from converting fibrinogen to fibrin, but it also allows the conversion of circulating protein C to APC
What activates Protein C complex?
Thrombin-thrombomodulin complex
Actions of Activated Protein C (APC)
Perhaps the most important player in decreasing both excessive inflammation and hypercoagulation
Anti-coagulant properties
After binding to cofactor Protein S it can inactivate FV and FVIII
Interacts directly with EPCR which enhances the conversion of protein C to APC by 5-fold
Interaction between APC and EPCR also blocks intracellular NF-kB which prevents cells from forming and releasing cytokines and chemokines
Inhibits adhesion of leukocytes to endothelial cells
Can inhibit endothelial cell apoptosis likely through binding to EPCR and possibly PAR-1 on the endothelial cells
Soluble EPCR-APC Complex
Soluble EPCR-APC complex can act at sites distant to the site of injury
Sepsis decreases the ability of EPCR-APC to become soluble and exert its anti-inflammatory and anti-coagulation effects systemically
Actions of Antithrombin
Antithrombotic protein
Binds to thrombin and prevents its pro-coagulant activation
Can also cause prostacyclin release from endothelial cells
Prostacyclin inhibits platelet activation and aggregation and has effects on endothelial cells such as reduction in tethering of neutrophils and decreased secretion of cytokines
When antithrombin binds to leukocytes through receptors like syndecan-4, it can block the interaction of these cells with endothelial cells
Accomplishes this by decreasing the expression of P-selectin, reducing activation of leukocytes and/or increase prostacyclin release
Procoagulant Components of Hemostasis
An activated vascular endothelium, activated platelets, and coagulation proteins
Abnormal or decreased fibrinolysis
Anticoagulant Components of Hemostasis
Antithrombin (AT), protein C and protein S (PC/PS), factor V, and prostacyclin (PGI2)
Normal fibrinolysis is antithrombotic
What are the two types of physiologic plasminogen activators?
Tissue plasminogen activator (tPA)
Urokinase plasminogen activator (uPA)
Tissue Plasminogen Activator
Released from endothelial cells in response to direct injury or following stimulation by thrombin
Urokinase Plasminogen Activator
Found in numerous cell types in the body and may have a greater role in extravascular fibrinolysis
What is the main regulator of the initiation phase of coagulation?
Tissue factor pathway inhibitor (TFPI)
What are the main regulators of the propagation phase of coagulation?
Antithrombin (AT) and activated protein C (aPC) pathways
Where are the three pools of TFPI in the body?
Within microvascular endothelial cells, stored in platelets, and circulating bound to lipoproteins
Actions of TFPI
Inactivates both the TF-FVIIa complex and FXa
Initially TFPI complexes with and inactivates FXa
The TFPI-FXa complex then binds to the TF-FVIIa complex forming a tetrameter than inactivates the TF-VIIa
What is the half-life of antithrombin in plasma?
Approximately 48-56 hours
May decrease to as little as 8-12 hours in sepsis induced consumptive coagulopathy
Actions of Antithrombin
Specific thrombin (FII) and FXa inhibitor, but also inhibits FVIIa, XIa, XIIa, and kallikrein
AT binds to serine protease coagulation factors via a serine-binding domain and the complex is removed by the reticuloendothelial system
When does AT function most effectively?
When its heparin binding domain is also occupied
Binding of cell surface heparin and other glycosaminoglycans (GAGs) results in a conformational change that allosterically potentiates AT enzymatic activity great than 1000 fold
Anti-Inflammatory Functions of AT Bound to GAGs
By damping down coagulation, it reduces the pro-inflammatory effects of the downstream coagulation factors such as thrombin
Also block expression of NF-kappa B and thereby reduces cytokine production and decreases leukocyte activation and adhesion
Also increases PGI2 production
Anti-inflammatory properites not realized when AT is bound to exogenously administered heparins
Where is Protein C synthesized?
In an inactive form in the liver, in a vitamin K dependent process
How is protein C activated?
Protein C is converted to its active form, aPC in the presence of the thrombin + thrombomodulin complex, a process which is facilitated by the endothelial protein C receptor
Action of Protein C
Once activated, protein C dissociated from the endothelial protein C receptor, binds to protein S and this complex inactivates FVa and FVIIIa
Anti-coagulant action
Inhibits plasminogen activator inhibitor-1 (PAI-1)
Profibrinolytic
Inhibits pro-inflammatory cytokine production and leukocyte activation likely via downregulation of NF-kB
Anti-inflammatory
Role of Thrombomodulin
Competes with the thrombin receptor for thrombin binding, minimizing the procoagulant and proinflammatory effects of thrombin
What activates thrombin-activatable fibrinolysis inhibitor (TAFI)?
Thrombin, thrombin-TM complexes, and plasmin
What is the action of TAFI?
Attenuates the conversion of plasminogen to plasmin
When thrombin is bound to thrombomodulin, activation of TAFI, and hence inhibition of fibrinolysis is increased 1250 times
What comprises Virchow’s triad?
Blood stasis
Hypercoagulability
Endothelial damage
What characterizes hypercoagulability?
Increased platelet activation, enhanced activation of coagulation factors, reduction of natural anticoagulant or inhibition of fibrinolysis
What can cause blood stasis?
Reduced blood velocity, reduced or turbulent blood flow, often caused by vascular or heart valve or chamber anomalies
What does endothelial injury revolve around?
Increased platelet activation and increased concentration of activated clotting factors
Composition of Venous Thrombus
Primarily composed of red blood cells embedded within a fibrin meshwork as they are formed at low shear stress
What is the most important part of arterial thrombus formation?
Platelet adhesion to the endothelium due to the predominance of high shear stress conditions
How does aspirin work?
Acts on the arachidonic acid pathway and irreversibly inhibits the COX pathways
COX1 and COX2 catalyze the conversion or arachidonic acid to PGH2, the precursor for several metabolites including thromboxane A2 (TXA2)
What is the function of thromboxane A2?
Enhances platelet function and promotes platelet aggregation and vasoconstriction
MOA of Clopidogrel
Thienopyridine that selectively inhibits ADP-induced platelet aggregation
Has no direct effects on arachidonic acid metabolism
Requires hepatic biotransformation to produce the active metabolite
MOA of Heparin
Complexes with and catalyzes the activity of the anticoagulant protein antithrombin
Action of the Heparin:Antithrombin Complex
Inhibits coagulation factors IIa (thrombin), IXa, Xa, Xia, and XIIa
IIa and Xa are most sensitive
By inactivating thrombin, heparin not only prevents fibrin formation, but inhibits thrombin-induced platelet activation and continued activation of coagulation factors V and VIII
What fraction of unfractionated heparin molecules contain the binding site for antithrombin?
1/3
The other 2/3 have minimal anticoagulant activity
Monitoring for Unfractionated Heparin Therapy
aPTT with an accepted therapeutic target range of 1.5-2.5 times the normal control aPTT value
How is the action of LMWH different from UFH?
LMWH has reduced anti-IIa activity relative to anti-Xa activity
MOA of LMWH
Binds to and catalyzes the activity of antithrombin
Can you monitor LMWH with aPTT?
No, prolonged aPTT seen with UFH primarily reflects inhibition of factor IIa
Inhibition of Xa has little effect on aPTT
What is the recommended monitoring for LMWH therapy?
Chromogenic assays of anti-factor Xa activity
Clinical Signs Associated with PTE
Variable, from mild dyspnea to sudden death, but revolve around respiratory signs such as hypoxemia, dyspnea, and hyperventilation
Hypoxemia is secondary to low V/Q matching
Arterial Blood Gas Findings with PTE
100% of dogs with PTE had an increased alveolar-arterial gradient and almost half had hyperventilation
Radiographic Patterns Described with PTE
Westermark sign - regional oligemia (hypoperfusion)
Hampton hump sign - shallow wedge-shaped opacity in the periphery of the lung with its base against the pleural surface
Routine Coagulation Profiles in PTE
Typically normal
D-dimers are more indicative that thrombosis has occurred
Treatment for PTE
Symptomatic treatment (oxygen, mechanical ventilation), treatment of the cause, and trhombophylaxis (UF, LMWH, or anti-XA) is usually provided
Effects of Thrombin on Platelets
Interact with PAR1 endothelium
In the presence of thrombin, platelets will bind more tightly to the endothelium
Activate platelets (degranulate alpha granules, activate GpIIb)
GpIIb likes to bind with the next platelet (II says that it needs the next platelet in line)
Scramblase moves phosphatidylserine (PS) from inner to outer platelet membrane → negatively charged phospholipid surface
What are FDPs?
Breakdown products of fibrin, crosslinked fibrin, and fibrinogen
Doesn’t give evidence that a clot was present
What are D-dimers?
Breakdown products of crosslinked fibrin
Tells you a clot was present
What are the actions of thrombin? Are they pro-clot or anti-clot?
Pro-clot
Activates platelets to propagate coagulation
Feeding back and activating FXI to amplify coagulation
Making fibrin and crosslinking it
Activates TAFI - inhibits fibrinolysis
Anti-clot
Activates tPA → plasmin
Binds to thrombomodulin → activates protein C
Thrombin activatable fibrinolysis inhibitor (TAFI)
Removes lysine residues from fibrin clot
Plasmin will not bind to the clot
Activated by thrombin
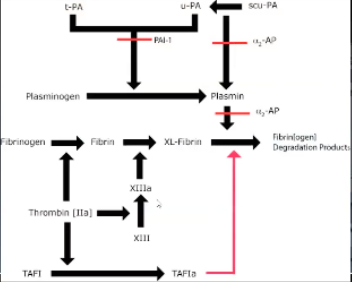
a-2 antiplasmin
Inhibits plasmin
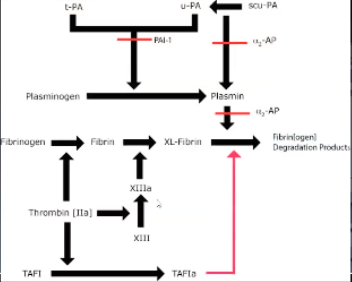
Plasminogen Activator-Inhibitor (PAI-1)
Inhibits activity of tPA/uPA → no plasmin formation
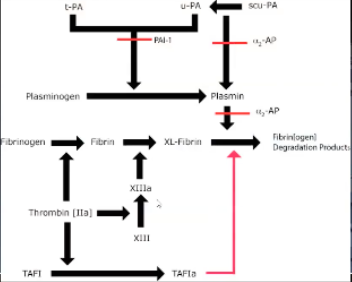
Tissue Factor Pathway Inhibitor (TFPI)
Inhibits Xa, TF-VIIa complex (extrinsic tenase complex)
Released from endothelium in response to heparin
Stored in platelets, endothelial cells
Circulating (bound to lipoproteins)
Protein C/Protein S
II binds to thrombomodulin on endothelial cell
Activates protein C which is bound to the endothelial protein C receptor
Becomes APC
Goes down the endothelium and activates protein S and have activated C and S
Inactivates V and VIII
Can remember because S kind of looks like 5 and can easily make an S into an 8
a-1 Protease Inhibitor
Inactivates Xa
a-2 Macroglobulin
Inactivates II
Heparan Sulfate
Proteoglycan on glycocalyx
Works with anti-thrombin
Inhibits II and X
Antithrombin
Made in the liver
Circulates in plasma
Inhibits II and X
Unfractionated Heparin
Potentiates antithrombin
AT → inhibits II and X
UFH binds to antithrombin and changes its shape so it can quickly and easily bind FX
Thrombin is a little bigger and doesn't find so well in the notch of antithrombin, with thrombin you need the tail of UFH to come and lock in thrombin
If you're binding FX and the long arm is still free the long arm that isn't locking in it can bind IX, XI, VII, and XII
Some inactivation of IX, XI, VII, XII
Will cause release of TFPI from the endothelium
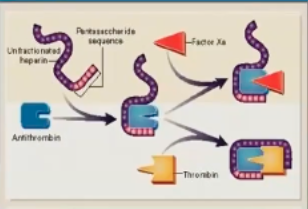
What % of UFH is the pentasaccharide that can activate AT?
20-50%
How is unfractionated heparin cleared?
Fast macrophages, endothelial cell binding → depolymerization
Slow clearance kidneys
Non linear clearance
What is the duration of action of unfractionated heparin?
Short duration of action (60 minutes)
Unfractionated Heparin Reversal
Protamine sulfate
Low Molecular Weight Heparin
Only binds to X
No long tail → no binding to II
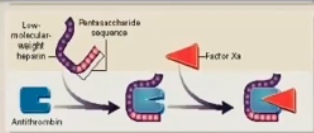
What fraction of the size of UFH is LMWH?
1/3 size (4-5000 daltons)
What % of the size of UFH is LMWH?
50-75%
Characteristics of LMWH
100% SQ bioavailability
More predictable effects
Longer half life (less binding macrophages, endothelium)
Clearance kidneys
Protamine zinc reverses 60% of anti-Xa activity (favors larger chains)
Ultra Low Molecular Weight Heparin
Fondaparinux
<3000 daltons
Only inhibit Xa + AT
No other effects of heparin
Only renal clearance
Therapeutic Monitoring for UFH
Monitor with aPTT
1.5-2.5x mean aPTT
Large multimers degrade faster
aPTT does not tell you about FX activity
Because it takes longer for small pieces to break down aPTT can be normal and still have FX inhibition
Univalent Direct Thrombin Inhibitors
e.g. argatroban, dabigatran
Non-covalent binding to thrombin
Reversible
Minimal bleeding risk and fast return to baseline
Can be reversed
Bivalent Direct Thrombin Inhibitors
e.g. lepirudine, desirudine, hirudin
Irreversible binding to thrombin
Cleaved by II once bound → slow return to function
Factor Xa Inhibitors
Primary site of action is the amplification stage of coagulation
No effects on platelets
Directly binds to Xa (bound and free)
Reversible
What is the major goal of platelet activation?
Activation of the integrin IIb/IIIa receptor → binds fibrinogen → platelets aggregate
Platelet GPCR Thrombin Receptors
PAR1
PAR4
PAR1 Ligand
Thrombin
PAR4 Ligand
Thrombin
Platelet ADP Receptors
P2Y1
P2Y12
P2Y1 Ligand
ADP
P2Y12 Ligand
ADP
Platelet Prostaglandin Family Receptors
TXA2
TXA2 Ligand
TXA2
Antiplatelet Drugs that Interfere with GPCRs and Their MOAs
COX Inhibitors (aspirin) - decrease thromboxane A2 production
Thienopyridines (clopidogrel, ticlopidine, prasugrel) - pro-drugs, irreversibly bind at P2Y12 receptors
Nucleoside analogs (cangrelor, ticagrelor) - reversibly block P2Y12 receptors
Platelet Leucine Rich Receptors
GPIb-IX-V
TLR2
TLR4
TLR9
Action of GPIb-IX-V Receptor
GPIb-IX-V is the major platelet receptor for vWF
Controls the first step in platelet adhesion, attaches the vWF on damaged subendothelium
In a high shear stress state, increased affinity for vWF, attachment cycles on and off rapidly so platelet can roll over the endothelial surface by temporary tethering
Have short cytoplasmic tails so participate in signal transduction and regulate binding affinity
Binding trigger intracellular signaling events, platelet activation, adhesion, aggregation
Platelet GP1b-IX-V Receptor Ligands
vWF, collagen, P-selectin