metals
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
Alloy
metallic substance of two or more elements (often non-stoichiometric)
Tie-Line (Isotherm) Rule
Used in binary phase diagrams to find compositions of two phases in a
two-phase region.
● Steps:
1. Draw tie-line at given temperature across the two-phase region.
2. Note intersections with phase boundaries.
3. Drop vertical lines to get compositions of liquid and solid phases.
The lever rule is a simple graphical method used in phase diagrams (especially in binary alloy systems) to determine:
1. The fraction (percentage) of each phase present in a two-phase region.
2. How much of each phase (e.g., liquid and solid) exists at a given temperature and composition
Iron wt% C ?
<0.008 wt% C → mostly ferrite.
Steel wt% C?
0.008–2.14 wt% C (usually <1% in practice).
Cast iron wt% C
2.14–6.70 wt% C
In the iron–carbon system, the eutectoid point happens at:
● 0.76 wt% Carbon
● 727°C
● At this point, austenite (γ) transforms into:
Austenite (γ)→Ferrite (α)+Cementite (Fe₃C)\text{Austenite (γ)} \rightarrow
\text{Ferrite (α)} + \text{Cementite (Fe₃C)}
forming a microstructure called pearlite.
Hypo-eutectoid Steel:
C amount ?
Cooling path:
Microstructure (forms what before reaching 727)
what happens at 727 ?
● Less carbon than 0.76% (e.g., 0.2%, 0.5%)
● Cooling path:
Austenite → Ferrite + Pearlite
🔍 Microstructure:
● Forms proeutectoid ferrite first (before reaching 727°C)
● Then, at 727°C, remaining austenite turns into pearlite
So, ferrite + pearlite structure
Hyper-eutectoid Steel:
C amount ?
Cooling path:
Microstructure (forms what before reaching 727)
what happens at 727 ?
● More carbon than 0.76%, but less than 2.11% (e.g., 1.0%)
● Cooling path:
Austenite → Cementite + Pearlite
🔍 Microstructure:
● Forms proeutectoid cementite first
● Then, at 727°C, remaining austenite turns into pearlite
So, cementite + pearlite structure
Photomicrograph of a 0.38 wt% C steel (hypoeutectoid) having a microstructure consisting of pearlite and proeutectoid ferrite.
Photomicrograph of a eutectoid steel showing the pearlite microstructure consisting of alternating layers of a-ferrite (the light phase) and Fe3C (thin layers most of which appear dark).
Photomicrograph of a 1.4 wt% C steel (hypereutectoid) having a microstructure consisting of a white proeutectoid cementite network surrounding the pearlite colonies.
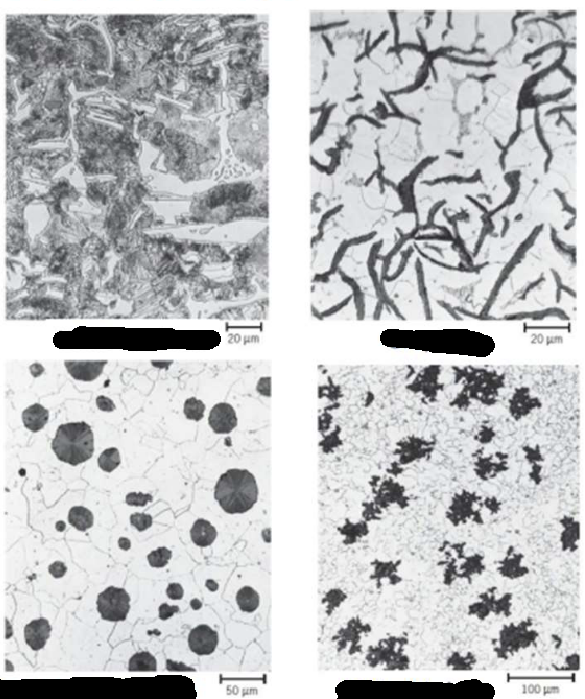
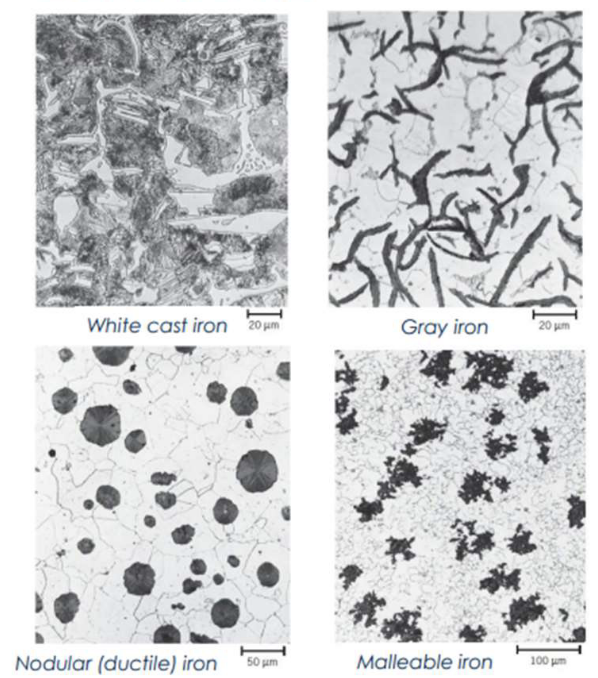
Cast Irons
General traits(what is it made of?):
why names “cast“?
melting range ?
General traits: carbon contents above 2.14 wt%: 3.0–4.5 wt% C, plus other
elements (like Si).
● Named cast iron because casting is the main shaping method.
● Lower melting range (1150–1300°C) than steel
Completely liquid at temperatures between approximately 1150°C and 1300°C
(lower than that of steel)..
different types of cast iron ?
White Cast Iron
Gray Cast Iron
Ductile (Spheroidal Graphite) Cast Iron
Malleable Cast Iron
White Cast Iron
○ Hard, brittle, contains iron carbide (Fe₃C).
○ Abrasion resistant; difficult to machine.
Gray Cast Iron:
○ 2–4% C + ~1% Si.
○ Graphite flakes cause brittleness in tension.
○ Stronger in compression.
○ High fluidity, low shrinkage → good for complex cast shapes.
○ Low cost; most widely used cast iron.
Ductile cast iron:
● Produced by adding Mg or Ce to molten iron to form spheroidal graphite.
● Matrix: Pearlite (as-cast) or ferrite (after ~700°C heat treatment).
● Mechanical properties:
○ Ferritic ductile iron: tensile strength 380–480 MPa, elongation 10–20%.
○ Much more ductile than gray cast iron; mechanical behavior approaches
steel.
Malleable Cast Iron
● Starts as white cast iron (graphite-free, carbon in Fe₃C).
● Heat-treated at 800–900°C in neutral atmosphere to decompose cementite →
temper carbon (graphite).
● Graphite appears in rosette/cluster form in a ferrite or pearlite matrix.
● Offers high strength and malleability, similar to nodular iron.
General Characteristics of Cast Irons
● Cannot be forged/rolled due to brittleness—only cast into shape.
● Advantages:
○ Low melting temperature
○ Good castability and machinability
○ Corrosion resistance
○ High compression strength (3–5× tensile strength)
● Weak in tension and bending; lose strength under high heat.
Ferrous Metallurgy 3 steps :

Blast Furnace
● Introduced in 1735 (coke-based).
● Reduces Fe₂O₃ (hematite) or Fe₃O₄ (magnetite) to metallic Fe.
● Outputs: molten iron + slag.
● Modern capacity: up to 10,000 tons/day.
Steel and Steelmaking
how is it categorized?
3 main steelmaking process ?
● Steel: Iron–carbon alloy (<1% C), may contain other elements.
● Categorized by carbon content: low, medium, high.
● 3 main steelmaking steps:
1. Impurity removal (oxidation)
2. Temperature control
3. Alloy element addition
Steelmaking Processes
then explain each
Bessemer
Basic Oxygen Steelmaking (BOS)
Electric Arc Furnace (EAF)
🧪 1. Bessemer Process (1856)
First method for mass-producing steel from pig iron.
Blows air (not pure O₂) through molten iron to remove carbon.
Now obsolete due to poor control over composition and impurity removal.
🌬 2. Basic Oxygen Steelmaking (BOS)
Modern primary method for bulk steel production
🔹 How It Works:
A converter (large steel vessel) is filled with molten pig iron + some scrap.
A lance blows pure oxygen at high speed over the molten metal.
Carbon + impurities (S, Si, P, Mn) oxidize and form gases or slag.
🔹 Key Features:
Fast: ~30–40 minutes per batch
Slag absorbs impurities and floats on top
Scrap is added to help control temperature
Produces mainly long products (e.g., rails, beams)
📊 Global Share (2018): ~71% of steel produced this way
⚡ 3. Electric Arc Furnace (EAF)
Used mainly for recycling scrap and producing high-quality steel
🔹 How It Works:
Large furnace uses electric arcs to melt scrap steel or direct reduced iron (DRI)
Can precisely control temperature and composition
Slag used to capture impurities
🔹 Key Features:
Very flexible: on/off as needed
Ideal for flat products (e.g., sheets, coils)
Lower CO₂ emissions if powered by renewable energy
📊 Global Share (2018): ~29%
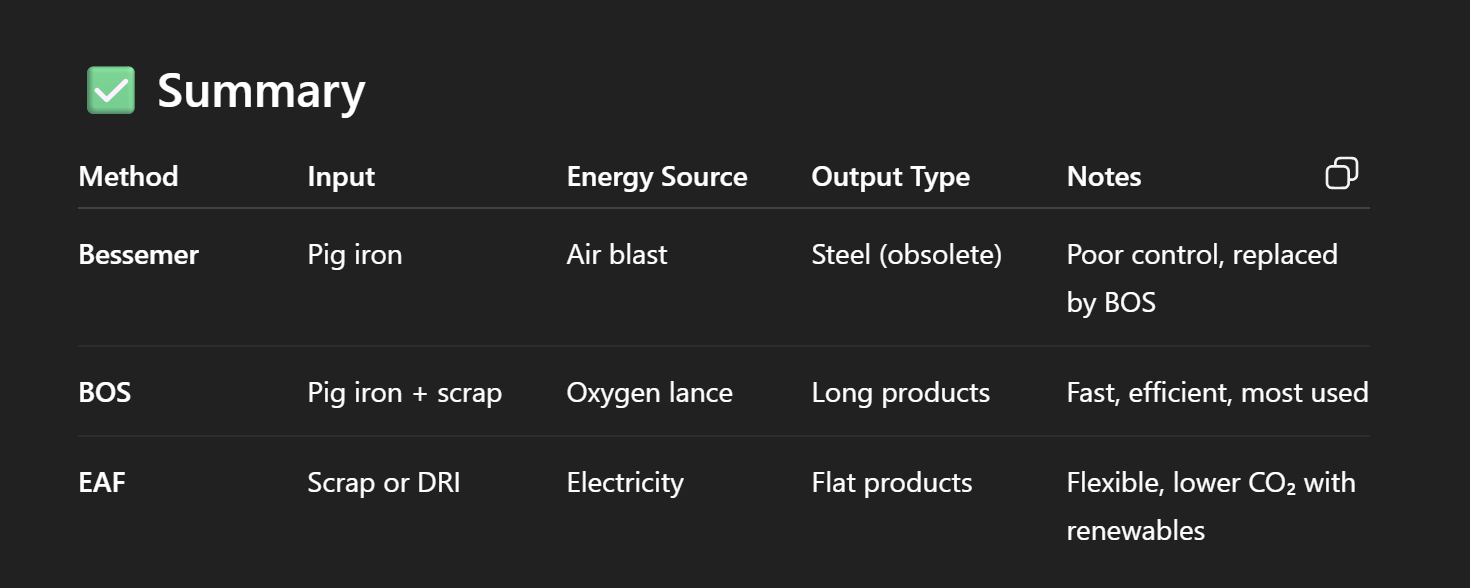
Forming Processes
● Continuous casting: Liquid metal solidified into billets/slabs.
● Roll forming:
○ Continuous shaping between rolls to form complex cross-sections.
○ Can produce open or closed profiles, even asymmetrical parts.
● Deep drawing:○ Sheet metal is deformed into a die by a punch while held in place to avoid
wrinkles.
○ Used to form curved or tubular parts, sometimes through multiple
stages
Steel in Buildings
1)Structural sections
2)Reinforcing bars
3)Sheet products
4)Non-structural steel

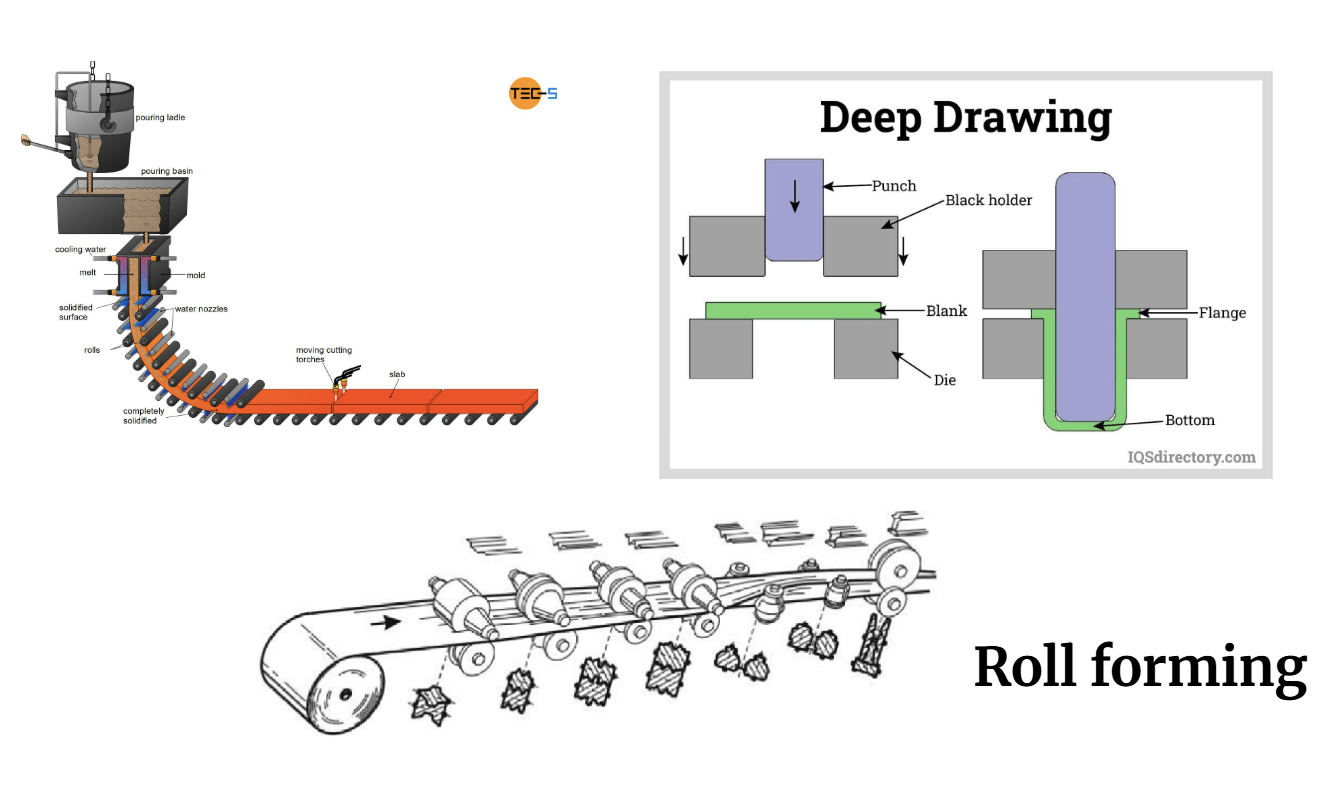
Steel in Infrastructure
1)Transport networks
2)Utilities (fuel/water/power)
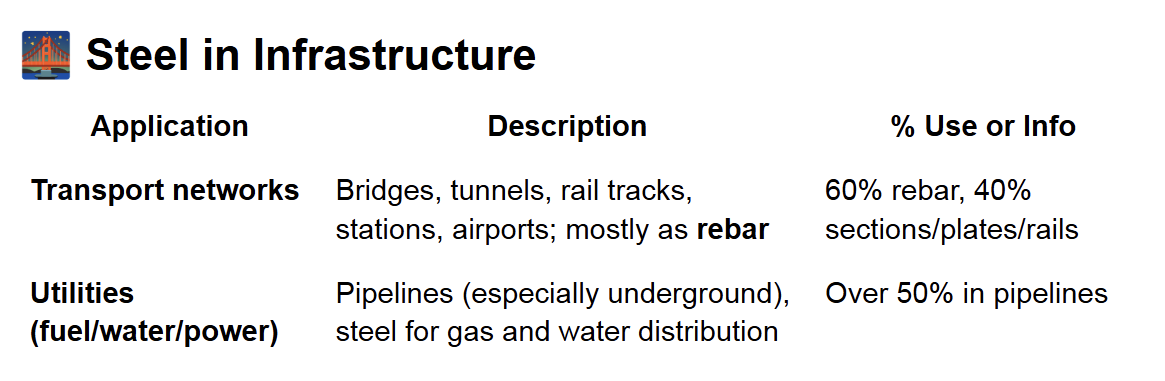
Types of Steel (by Carbon Content)
and in general
Low-carbon steel
Medium-carbon steel
High-carbon steel
Stainless steel
HSLA steel
Weathering steel (COR-TEN)
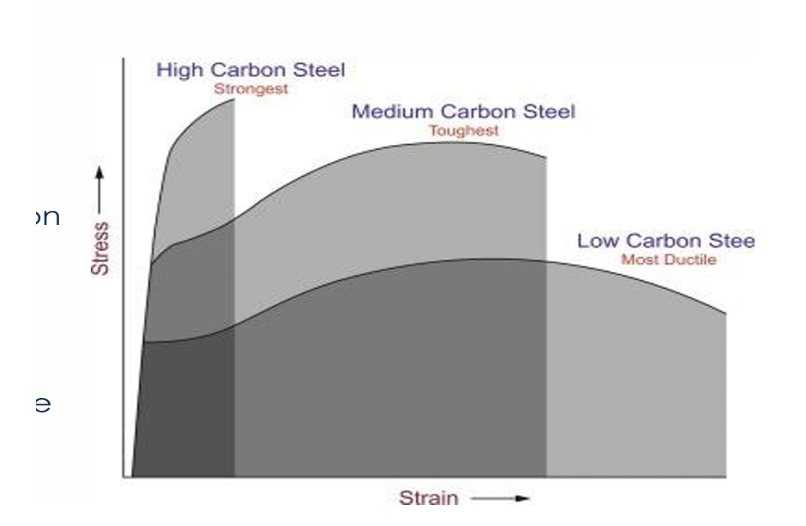
Steel (by Carbon Content)
toughest?
strongest ?
most ductile ?
Stainless Steel
≥10.5% Chromium, ≤1.2% Carbon → Highly corrosion-resistant
(e.g., kitchenware, medical tools).
HSLA / Microalloyed Steel
○ Alloyed with Cu, V, Ni, Mo (up to ~10%)
○ Stronger than plain carbon steel
○ Good ductility and formability
○ Tensile strength often > 480 MPa
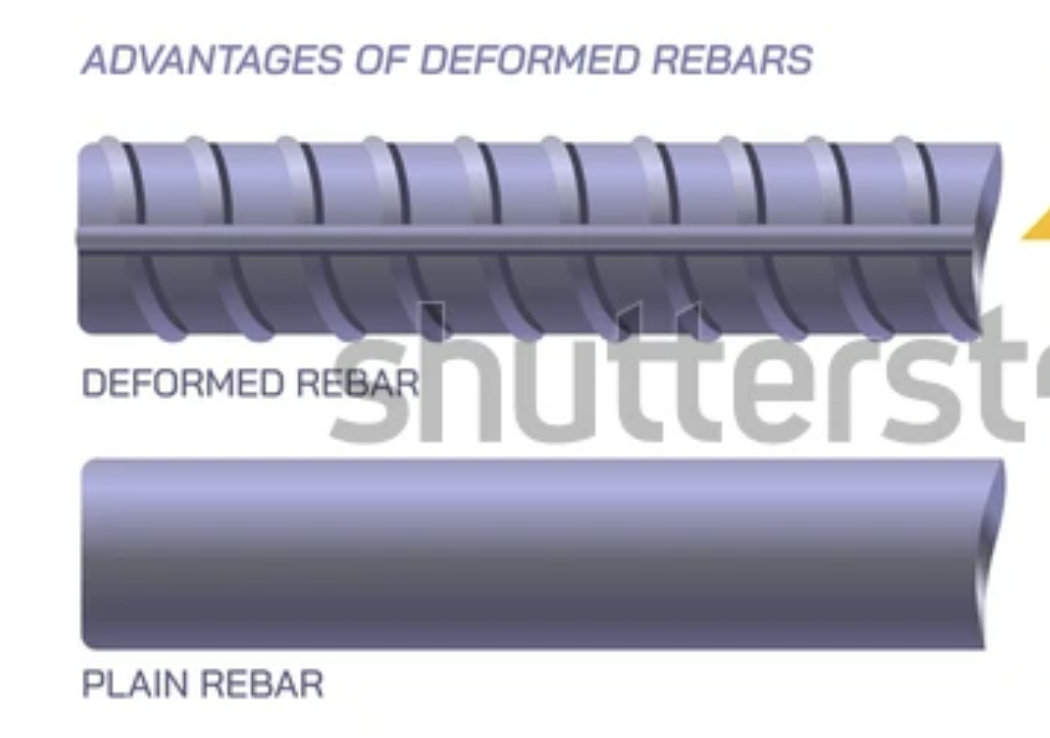
Reinforcing Steel (Rebar)
Smooth rebar
: ~250 MPa Basic use, less grip in concrete
Deformed rebar:
~500MPa Ridges improve bond with concrete, stronger, used in structural elements
Carbon Effect on Steel Properties
● ↑ Carbon → ↑ Strength and hardness, ↓ Ductility and weldability
● Elastic modulus (stiffness): NOT affected by carbon content
● Toughness (balance of strength + ductility): Best in medium-carbon steels
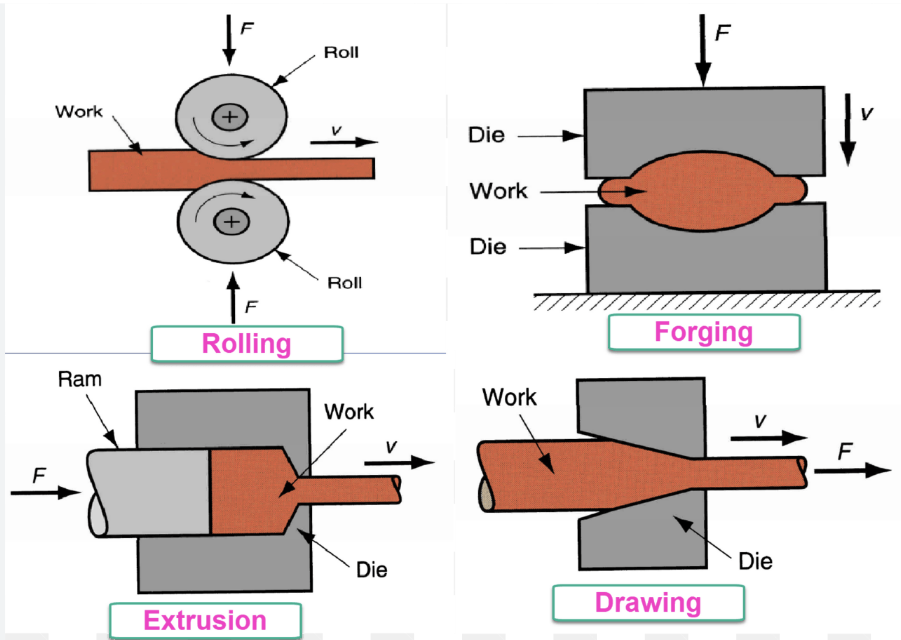
Forming Operations (Shape change via plastic
deformation)
🔹 Main Processes:
● Forging: Compressive forces shape metal (hammers/dies)
● Rolling: Reduces thickness via rollers
● Extrusion: Metal forced through die to produce long shapes
● Drawing: Pulls metal into wires or rods
Hot Working vs Cold Working
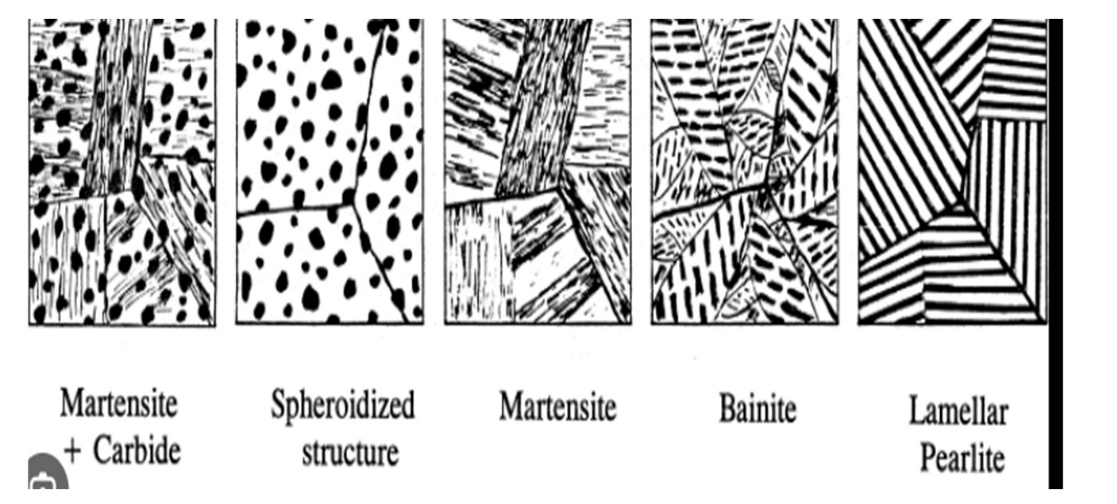
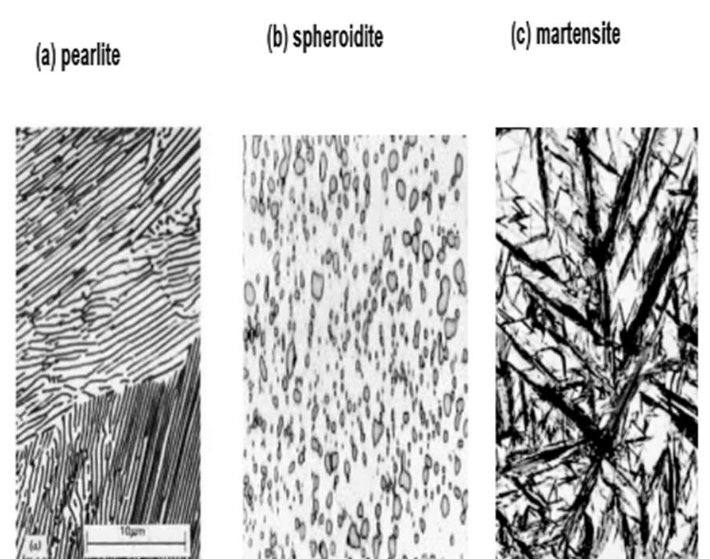
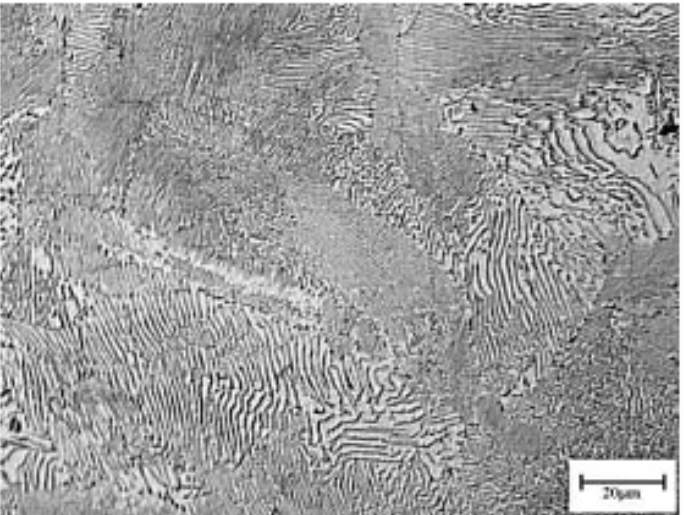
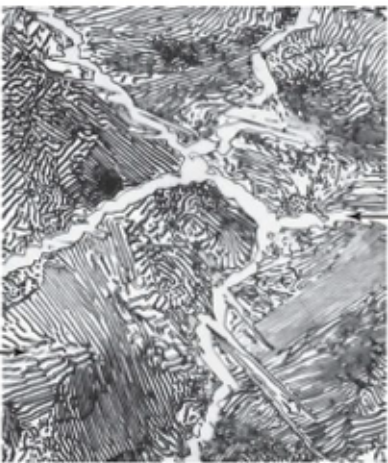
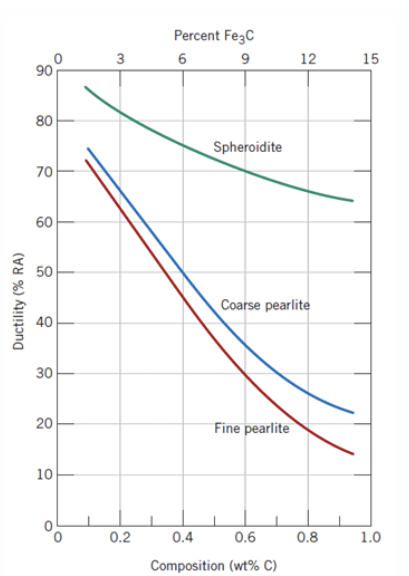
Pearlite
● Alternating layers of α-ferrite + Fe₃C (cementite)
● Coarse Pearlite: Forms near eutectoid temp. (thicker layers)
● Fine Pearlite: Forms at lower temps. (thinner layers, stronger)
coarse or fine pearlite ? which one is more ductile? why?
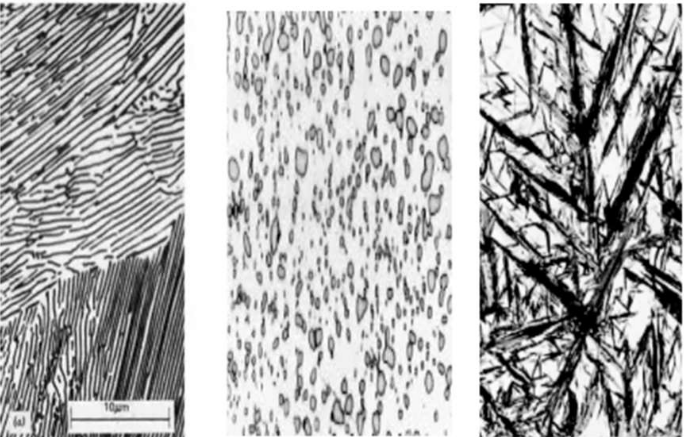
Coarse pearlite is more ductile than fine pearlite. This behaviour results from the greater restriction to plastic deformation of the fine pearlite.
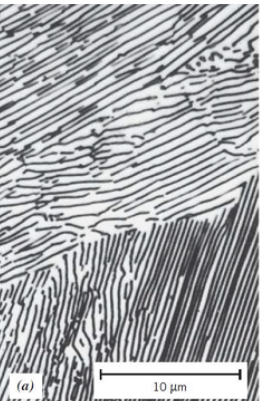
coarse pearlite

fine pearlite
Bainite
● Forms at temps below those for pearlite
● Microstructure: ferrite + elongated cementite
● Shape: plates or needles
● Stronger and harder than pearlite
● Forms competitively with pearlite (can’t get both without reheating)
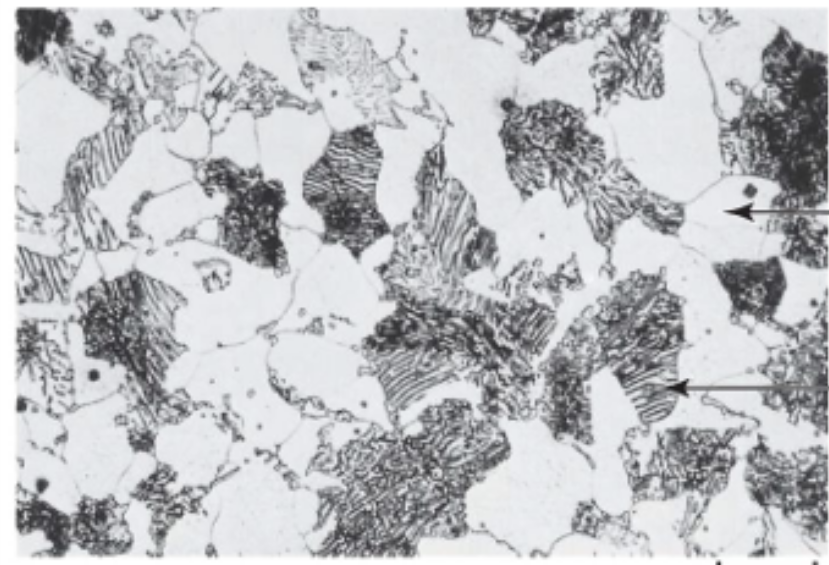
Spheroidite
● Formed by prolonged heating below eutectoid temp (~700°C for 18–24 hrs)
● Structure: Spherical Fe₃C in ferrite matrix
● Goal: Soften hardened steel for machining (max ductility, min hardness)
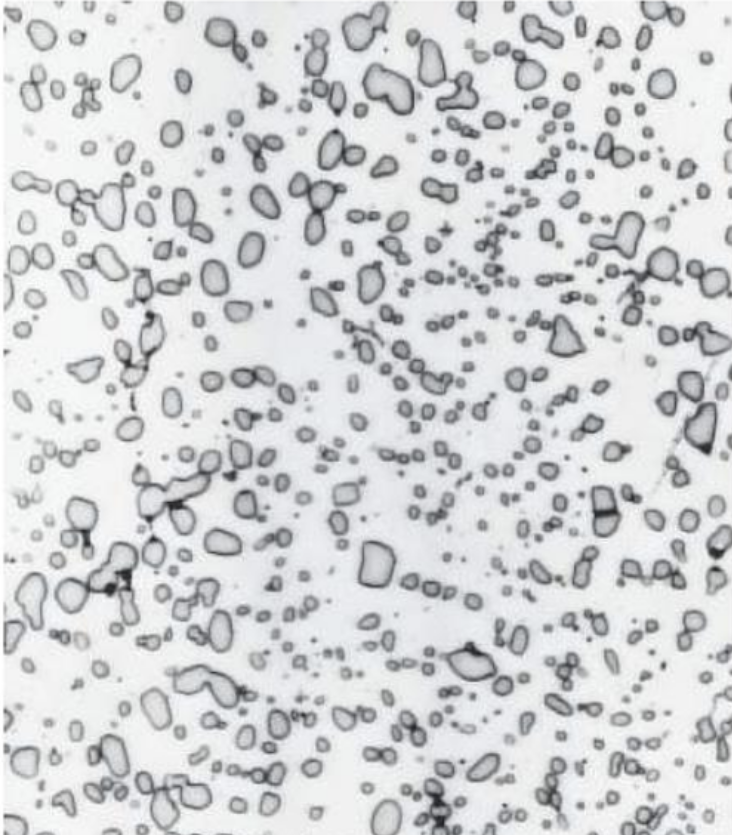
Spheroidite
Martensite
● Forms by very rapid cooling (quenching) from austenite
● Diffusionless transformation
● Structure: body-centered tetragonal (BCT)
● Extremely hard and brittle
● Transformation starts at M(start) and proceeds through M(50%), M(90%)
● Athermal: Depends on temperature, not time

Martensite (needle-shape grains)
Austenite
(white regions. It failed to transform due the rapid cooling)
TTT and CCT Diagrams
critical cooling rate
Define critical cooling rate for 100% martensite formation
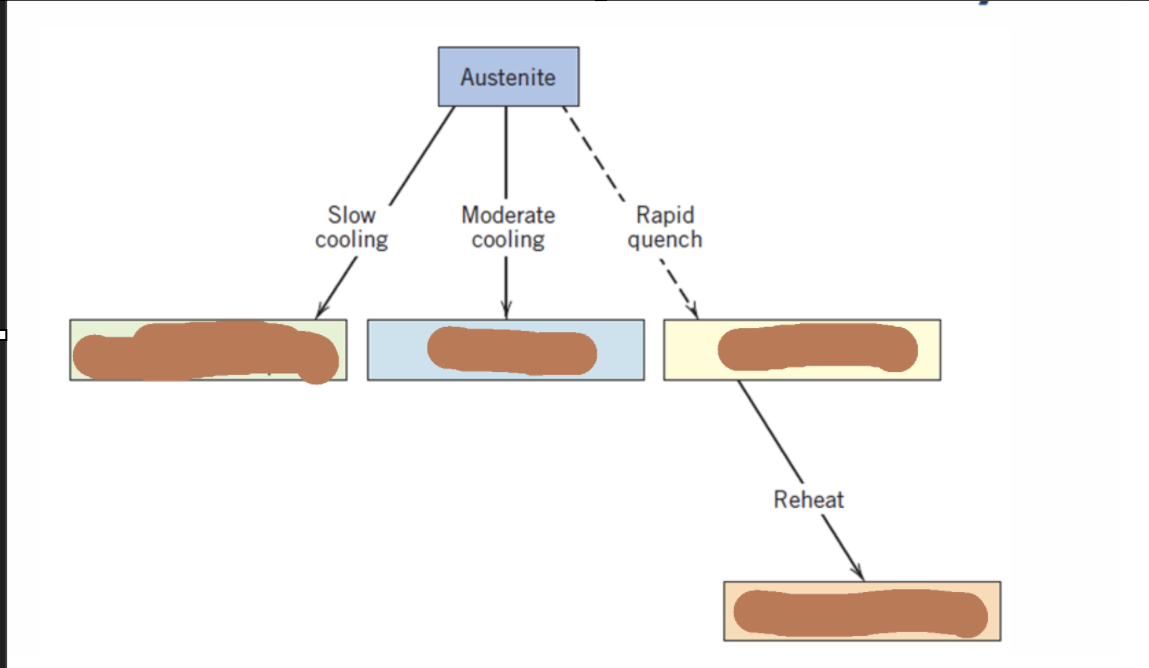
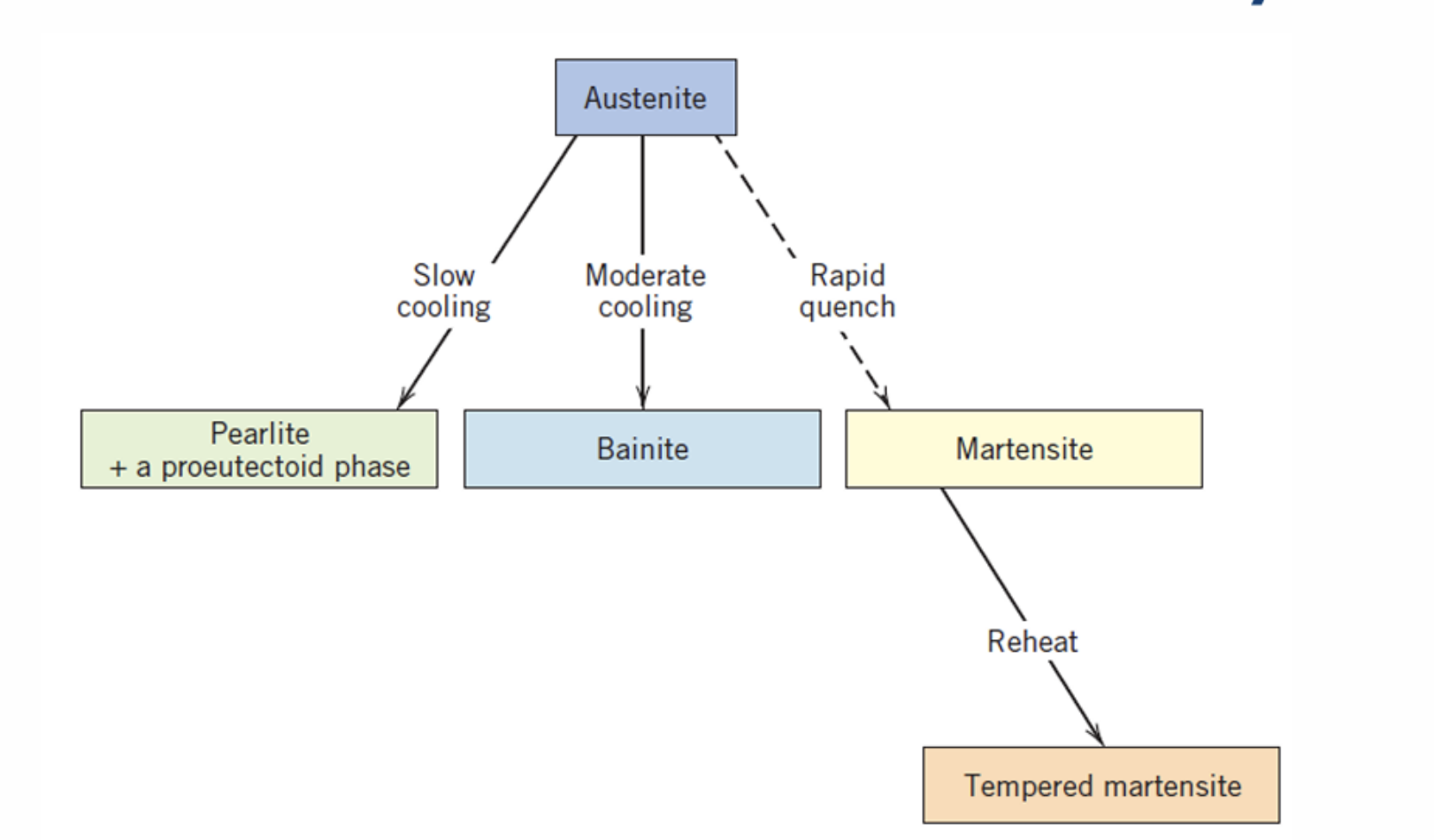
Cementite
Cementite (Fe₃C) is much harder but more brittle than ferrite.
● Increasing cementite content:
○ Increases hardness and strength.
○ Decreases ductility and toughness (impact energy).
● Cementite in fine pearlite reinforces ferrite more effectively than in coarse
pearlite due to:
○ Greater phase boundary area (impedes dislocation motion).
○ Stronger restriction on plastic deformation.
Isothermal vs Continuous Cooling
● Isothermal heat treatment: not practical due to need for rapid cooling and
holding at elevated temp.
● Continuous Cooling Transformation (C-C-T) diagram:
○ Isothermal curves shift to longer times and lower temps.
○ Used for most industrial processes.
Critical quenching rate
○ Minimum rate to achieve fully martensitic structure.
○ Slower rates yield a mix of pearlite + martensite or just pearlite.
Martensite
Hardest and most brittle steel microstructure.
○ Strength not due to structure but carbon hindering dislocation motion +
few slip systems in BCT.
○ As-quenched martensite is usually too brittle for use.
Tempering Martensite
○ Heat martensite below eutectoid temperature (200–650°C).
○ Produces tempered martensite: α-ferrite matrix with cementite particles.
○ Improves ductility & toughness, relieves quenching stresses.
○ Higher tempering temp → larger cementite particles → softer,
weaker, but tougher.
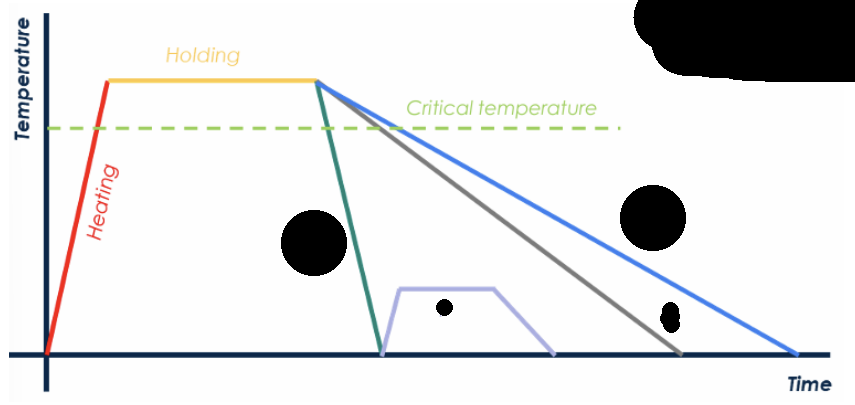
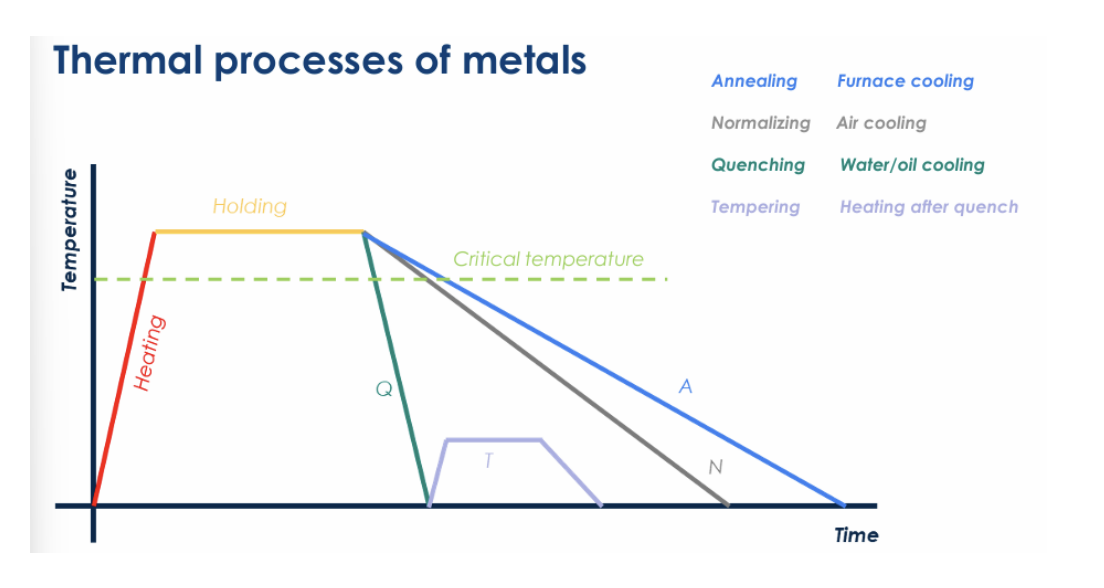
name each color and describe
Weathering Steel
● COR-TEN: trademarked by U.S. Steel (CORrosion resistance + TENsile
strength).
● Forms stable, protective oxide layer that regenerates in weather.
● Main alloying element: Copper.
● Not fully rust-proof—poor drainage increases corrosion.
● Natural oxidation takes ~6 months, but can be accelerated to ~1 hour with
surface treatments.